| One of the simplest mechanisms in physical optics is scattering. From the viewpoint of the sender, scattering randomizes light by reflecting it in myriad directions. From the standpoint of the viewer, it randomizes light by combining light from many sources. |

| Several scattering mechanisms are evident in this sunset scene. On the average, skylight is singly scattered, that is, only scattered once. The scattering particles are tiny enough to scatter short wavelengths more than long. Blue light is scattered more than red, making light scattered from the sky blue. When the path length through the atmosphere is long, as at sunset, everything but red is scattered and only red light reaches our eyes, making the sun red. The rays radiating from the sun, called crepuscular rays, are due to larger particles in the air causing a certain amount of multiple scattering as well. The few clouds are dark at this viewing angle because they scatter incident sunlight, allowing little to penetrate the clouds, although thin parts of the clouds transmit light and appear bright. |

| The one color that is almost always associated with a specific mechanism is white. Virtually all whites are due to multiple scattering; off grain boundaries as in sugar and salt, cellulose fibers as in paper, or colloidal fat globules as in milk. (Some people assert that black and white are not strictly colors. My view is that anything the eye and brain process, using the same information processing that they use to interpret colors, is a color.) |

| The white of clouds is due to multiple scattering off water droplets or ice crystals. |

| The white of snow is due to multiple scattering off ice crystals. |
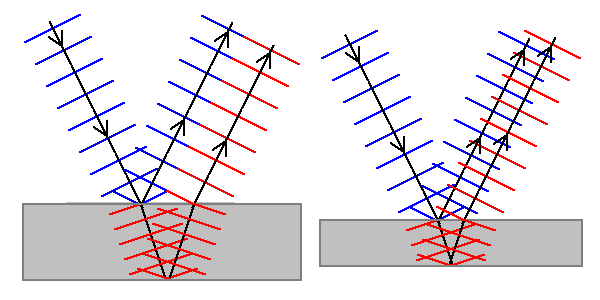
| When light beams are split and recombined, they can undergo interference. Thin film interference occurs when light reflected from the two surfaces of a thin film interferes. Some wavelengths of light are intensified, others cancelled out. |
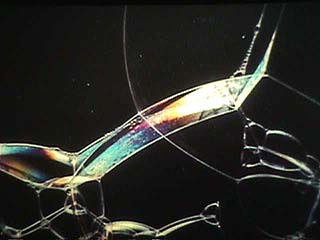
| The colors of soap bubbles are a classic example of thin-film interference. Just before a soap film breaks it stops showing color because it becomes too thin to produce interference. |

| Ore minerals frequently have iridescent films because of a thin layer of alteration on the surface. |

| This Roman plate has developed a spectacular iridescent finish because of a thin film of oxide on its glaze. The play of colors on an oil slick are also due to thin-film interference. |

| Shells have softer and more subdued interference colors because of multiple-layer interference. Scattering and superposition of numerous wavelengths softens the colors. This slide shows a 19th-century button-making apparatus that used Mississippi River mussel shells for raw materials. |
 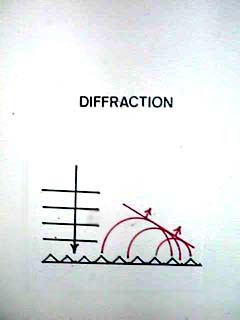
| Diffraction is closely related to interference. It occurs when regularly-spaced structures scatter light, and certain wavelengths of light constructively interfere (are intensified) while others destructively interfere (cancel out). |

| The familiar rainbow play of colors on a compact disk is due to tiny pits roughly a wavelength of light apart. Phonograph records (remember those?) produce crude diffraction effects because of the much wider spacings of their grooves. |

| Regular, closely spaced structures are common in the biological world and are responsible for diffraction colors like peacock wings or the brilliant blue of this morpho butterfly. |

| Beetles are almost as popular among insect collectors as butterflies, partly because of their bright diffraction colors. |

| The play of color in opal is also due to diffraction of a most amazing sort. |

| Technically, opal is not a mineral, but a mineraloid, a non-crystalline misture of silica and water. on the microscopic scale, opal is made of tiny colloidal silica spheres. When they are regular in size and stacking, they produce the colors of precious opal. |
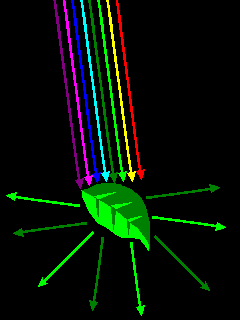
| Most colors are due to various absorption mechanisms, where some wavelengths of light are absorbed but others are not. |
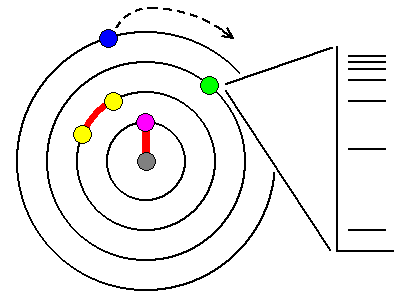
| Absorption is due to interactions between photons and electrons. The outermost electrons in atoms are often lost to ionization. Innermost electrons are too tightly bound to the nucleus to be excited by anything short of X-rays. Most orbital electrons are paired and too tightly bound to be affected by visible light. Only unpaired electrons are loosely enough bound to interact with visible light photons. |

| Individual atoms emit and absorb characteristic wavelengths of light. This fact is the basis of spectroscopy, and also causes most of the colors seen in fireworks (though chemical reactions during combustion also play a role.) |
| In their most common ionization states, only the transition metals have unpaired electrons. These are thus common color-formers in minerals. |
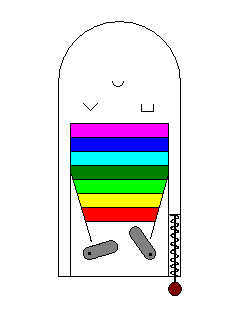
| We can liken electron excitation to a pinball game. The paddles supply energy, the way a photon does to an electron, and the various targets on the board correspond to the excitation levels of the electron. Materials that have no excitation levels in the range of energies corresponding to visible light won't interact with visible light and will be colorless. |
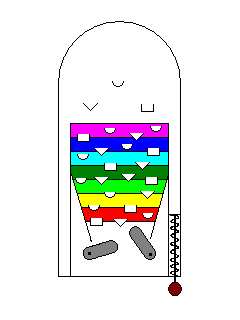
| If there are lots of excitation states in the visible range the material will absorb most of the incident light and will be dark and opaque. |
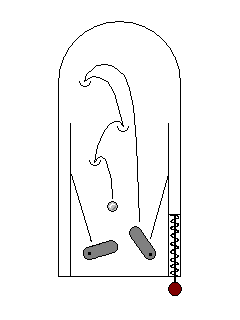
| Sometimes electrons can be excited by high-energy photons (ultraviolet) but fall back to their ground state through several steps in the visible range. This is fluorescence. Your washday miracle gets clothes white not only by getting them clean but by adding a dye that glows blue-white in solar ultraviolet light. |
| Sometimes an electron will stay excited for a long time before falling back to its ground state. This is phosphorescence. "Glow-in-the-dark" plastics are phosphorescent. |
| Sometimes an electron can be jarred out of an excited state by a mild mechanical disturbance, like scratching or breaking. This is called triboluminescence. Hard candies sometimes do this when broken - the luminescence seems to be due to nitrogen atoms from the air adsorbed onto the surface. A related effect, thermoluminescence, is the emission of light under very mild heating, long before the material would be incandescent. |
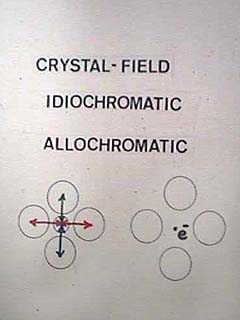 | Colors due to specific atoms in a material are called crystal-field colors. The physics is very complex in detail because neighboring atoms distort the energy levels of orbital electrons. Colors are termed idiochromatic if they are due to an intrinsic ingredient of the material, and allochromatic if due to an impurity. Sometimes lone electons occupy vacancies in a crystal. These, being loosely bound and unpaired, are very effective at interacting with visible light. |
Idiochromatic Colors

| Cobalt compounds, like the mineral erythrite, tend to be pink, purple, or blue. |

| Manganese tends to produce pink colors. This specimen of rhodochrosite, manganese carbonate, is unusually vivid. |

| One of the most familiar idiochromatic coloring agents in minerals is copper, which is noted for its blue and green colors. |

| Divalent iron, frequently substituting for magnesium, produces the dark green or black colors of many silicate minerals. Tremolite, a magnesium amphibole, is white, whereas actinolite, essentially the same mineral but with iron substituting for magnesium, is green. There is a continuous compositional range between the two minerals. |
Allochromatic Colors

| Pure quartz is clear, but can be any color at all due to allochromatic coloring agents. |

| Purple quartz, or amethyst, owes its color to |

| As testimony to how complex crystal-field interactions can be, chromium is an allochromatic coloring agent responsible for both the green of emerald and the red of ruby. |
Charge Transfer and Molecular Orbitals
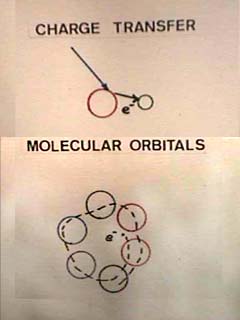 | Many colors are due to interactions between atoms. Charge-transfer colors result when photons transfer an electron from one atom to another. Molecular orbitals, common in organic materials, occur when electrons are bound collectively to entire molecules rather than single atoms. |

| Kyanite is aluminum silicate, and ought to be colorless. Trace impurities of ferrous iron substitute for the aluminum and give it the bluish hue that gives the mineral its characteristic field appearance and name. Charge transfer occurs between iron and oxygen. |

| Chromates derive their intense hues from charge transfers between chromium and oxygen. This is the rare mineral crocoite, lead chromate |

| Iron is far and away the dominant coloring agent in rocks and soils. All the colors in this picture are due to iron. Divalent iron makes silicate minerals dark green or black. Trivalent iron participates in charge transfers that are strongest in the ultraviolet, but somewhat effective in the visible range. Thus the blue end of the spectrum is absorbed and the familiar "earth tone" colors result. |

| In the last century, we have learned how to synthesize compounds with molecular orbital colors. |
| Some chemical reactions among organic chemicals give off light, like in this firefly. We have recently learned to synthesize chemiluminescence. |

| In the mid 19th century, we began to learn how to create molecular orbital colors ourselves, though we learned how to make the colors before we really understood the mechanism. It suddenly became a colorful world. |
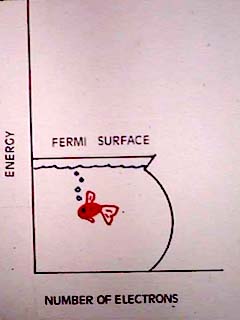 | In metals, all free electrons are alike, a condition called degeneracy. The Pauli Exclusion Principle causes these electrons to assume all energy levels up to some maximum value. Above that maximum energy, called the Fermi Surface, are unfilled energy levels. Any visible photon can find an electron to excite. Thus metals are opaque. The excited electron falls quickly back to its ground state, emitting the same wavelength it absorbed. Thus metals are highly reflective. |

| Most metals, like this silver, have energy levels uniformly-enough distributed that all wavelengths are equally affected, so the metals are neutral in color. |

| Some metals, like copper or the gold shown here, have unevenly distributed available energy levels. Gold and copper cannot interact with high-energy photons quite as efficiently as low energy, and thus reflect the red end of the spectrum more efficiently. A corollary is that blue photons can penetrate gold more easily than red photons, and in fact a very thin sheet of gold leaf is transparent to blue light. A scene viewed through thin gold leaf looks blue-green. |
| In semiconductors, there is an energy gap between electrons bound to atoms and the energies of free electons. These materials are normally nonconductive, but can be made conductive by supplying the right energy. Inducing conduction in semiconductors is the whole basis of solid-state electronics. Some semiconductors have such narrow band gaps that any visible photon can bridge them. They therefore behave optically much like metals. |

| Pyrite (iron sulfide) is a narrow band gap semiconductor, as are many metallic sulfide minerals. |

| Stibnite (antimony sulfide), noted for its spectacular crystals, is another. |
| Semiconductors with intermediate-width band gaps absorb high-energy wavelengths. They typically appear red or yellow as a consequence. |

| Sulfur is one such material. |

| Other intermediate-width band gap semiconductors include the sulfides of arsenic, realgar (red) and orpiment (yellow), and the sulfide of mercury, cinnabar (red). |
| Semiconductors with wide band gaps absorb no wavelengths in the visible light range and are colorless. |

| Diamond is one such semiconductor. |
| Impurities in diamond can introduce color. Atoms that introduce energy levels capable of accepting a valence electron are termed acceptors. |
 |
Other impurity atoms have electrons capable of jumping to the conduction band. These are termed donors. |

| Our colorful world owes its beauty to myriad processes on the atomic level that have their own kind of beauty. The green grass and red sumacs owe their color to molecular orbitals. The sky colors are due mostly to scattering. |