Ammonia
Steven Dutch, Professor Emeritus, Natural and Applied Sciences,
Universityof Wisconsin - Green Bay
Ammonia is a gas on earth but likely to be a common solid in the outer solar system. The molecules are approximately in a cubic close packed arrangement, but the asymmetry of the molecules reduces the symmetry to 2/m3, the same as pyrite. In the diagram below, nitrogen is green and hydrogen pink, with lighter colors denoting background molecules. The unit cell on the right shows the two-fold screw axes. The solitary axes extend into the plane of the diagram. Horizontal axes are indicated with purple lines and symbols at the ends (in reality, of course, the axes extend indefinitely).
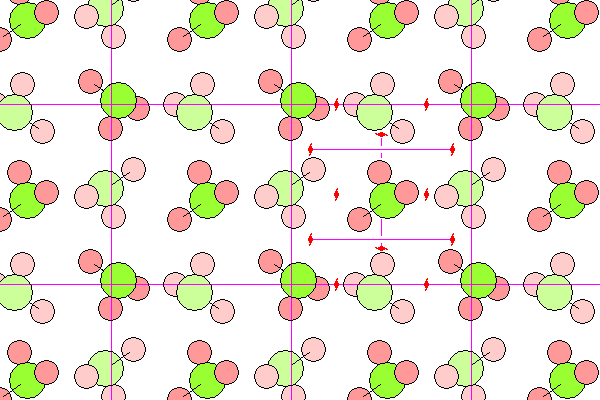
Like carbon, nitrogen has hybrid sp orbitals in a tetrahedral arrangement, but only three are shared. So an ammonia molecule looks like a methane molecule with a hydrogen missing. The asymmetry makes it polar, and a hypothetical alternative to water in exobiology.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 18 April, 2011, Last Update