Anhydrite
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
For a mineral with a simple composition, anhydrite (CaSO4) is surprisingly hard to visualize. The simple sulfate minerals have sulfate tetrahedra in a regular arrangement, and the cations have oddly shaped coordination polyhedra since they fill the interstices. The oddball polyhedra result because the tetrahedral oxygen arrangements around the sulfate groups won't permit a more classical coordination arrangement.
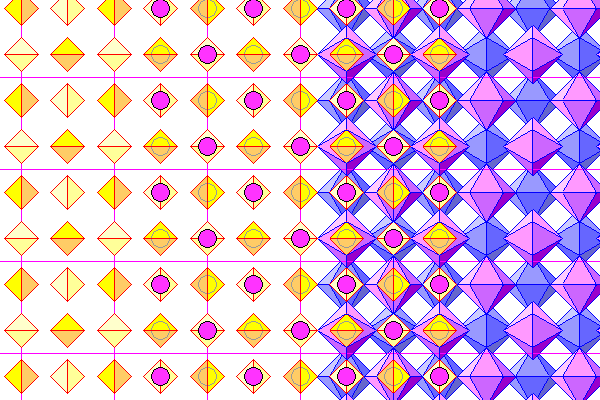
Above is a view down the c axis. The unit cell is almost square, differing from a square by less than 0.1%. From left to right, we see sulfate tetrahedra, tetrahedra with calcium atoms (purple), tetrahedra with atoms in foreground and coordination polyhedra behind, and finally only coordination polyhedra. Foreground polyhedra are reddish violet and rear ones bluish.
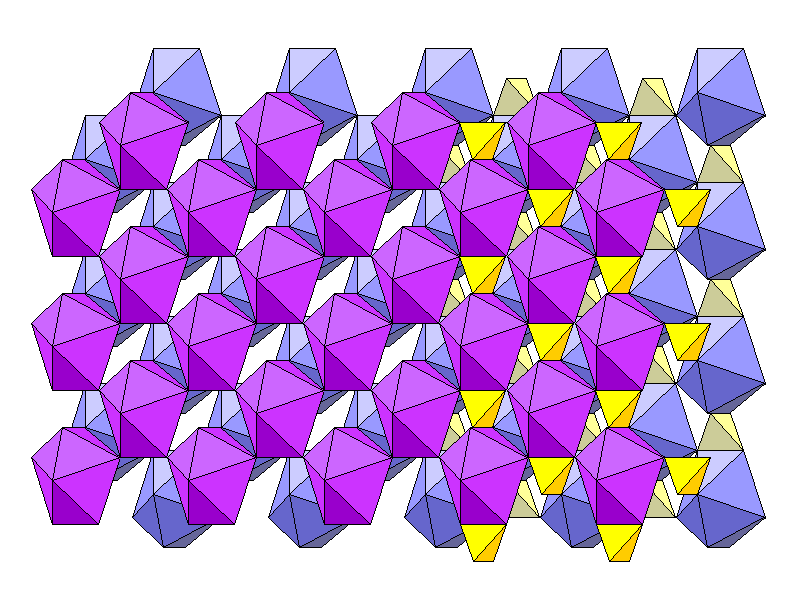
Above is an oblique view with the c axis vertical. Foreground coordination polyhedra are reddish violet and rear ones bluish. Each has an oxygen atom at each vertex. Calcium atoms are in eight-fold coordination with oxygen, but the coordination polyhedron is not the normal cube. Instead it has 12 triangular faces. One way to imagine it is to make a shape of two pentagonal pyramids, joined base to base but with two edges not attached. Squeeze the pyramids from the sides to open a gap along the unjoined edges, then insert a pair of faces in the gap. This shape is a snub disphenoid. Sulfate tetrahedra are shown at right, with foreground tetrahedra in bold colors.
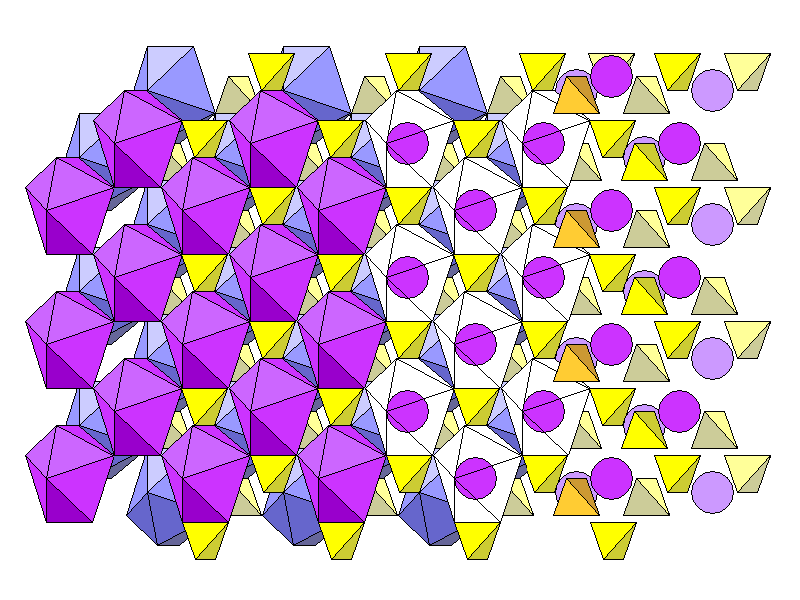
From left to right, above, are calcium coordination polyhedra, polyhedra with sulfate tetrahedra, polyhedra shown transparent to reveal the central calcium atoms, and finally calcium atoms and tetrahedra alone. Three levels of tetrahedra and two levels of calcium atoms are shown. From foreground to rear, we have tetrahedra in gold, calcium atoms in magenta, tetrahedra in bright yellow, calcium atoms in light purple, and tetrahedra in pale yellow.
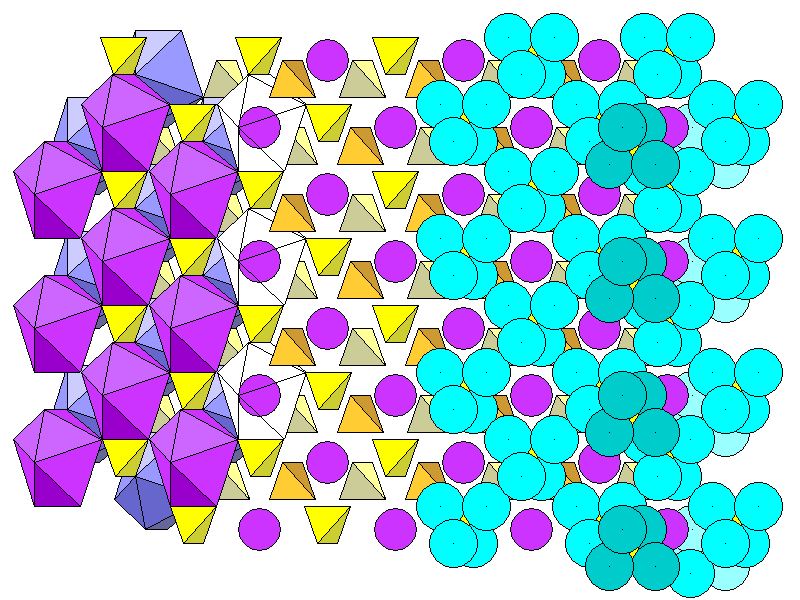
Finally, the actual arrangements of atoms. From left to right: calcium polyhedra and sulfate tetrahedra, atoms and tetrahedra, and atoms. The oxygen atoms in the tetrahedra are shown in blue. On the left, we can see that tetrahedra above and below the calcium atoms have two oxygens in coordination, while the tetrahedra on either side have one. At right the foreground (dark) and rearmost (light) tetrahedra are also shown.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 28 March 2011, Last Update