Barrovian Facies Metamorphism of carbonate Rocks
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
The Barrovian facies series, named for George Barrow, who described them around 1900,is the "normal" metamorphic facies series. These are the rocks formed in atypical geothermal gradient of about 25 C per km. The typical pressures under which theserocks form are 5-10 kb (mid-crustal depths). All temperatures are approximate and dependent on pressure.
Low Temperature
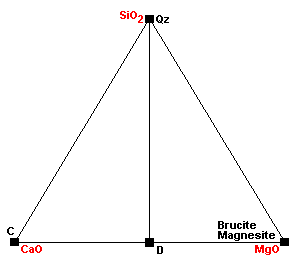 | The normal sedimentary assemblage in carbonate rocks. |
400 C
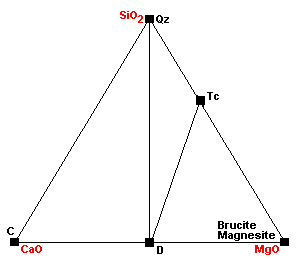 | Around 400 C, talc forms via the reaction 3Mag + 4Qz + H2O = Tc + 3CO2. |
450 C
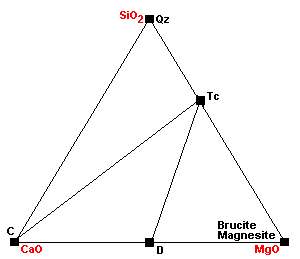 | Around 450 C, dolomite ceases to coexist with quartz: 3Dol + 4Qz + H2O = Tc + 3Cal + 3CO2. This is the same reaction as above, except this one extracts the magnesium component from dolomite. |
500-550 C
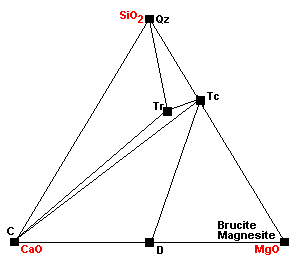 | At about 500 C, talc, calcite and quartz react to form tremolite. Calcite can coexist with talc only under extremely restricted conditions. |
Greenschist Facies
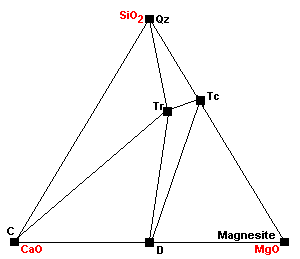 | The marginal compatibility of calcite and talc ends completely as calcite plus talc react to give tremolite plus dolomite. |
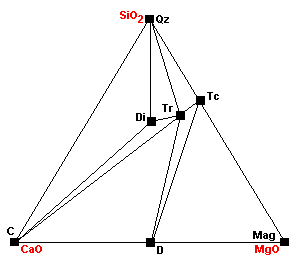 | About 520 C diopside begins to form: Tr + 3Cal + Qz = 5Di + 3CO2 + H2O |
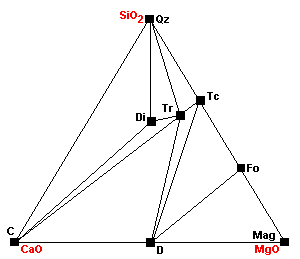 | Forsterite appears: Tc + 5Mag = 4Fo + 5CO2 + H2O. Note that talc is becoming progressively less compatible with carbonates. |
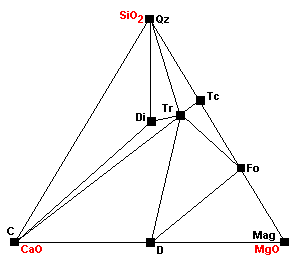 | Dolomite and talc can no longer coexist as dolomite plus talc react to give tremolite plus forsterite. Note that talc now cannot coexist with any carbonates. |
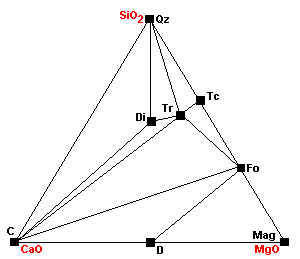 | Around 540 C Tr + 11Dol = 8Fo + 13Cal + 9CO2 + H2O Note how the stability fields of dolomite are shrinking with each reaction. |
600-700 C
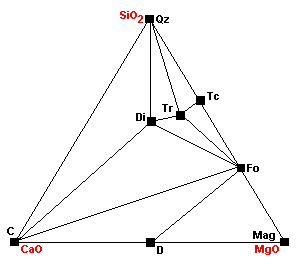 | Around 600 C 3Tr + 5 Cal = 11Di + 2Fo + 5CO2 + 3H2O |
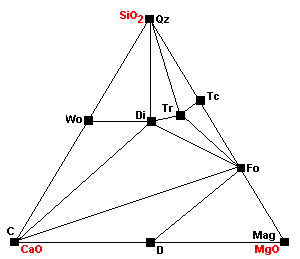 | About 640 C at 1 kb, or 680 at 2kb, wollastonite forms: Cal + Qz = Wo + CO2 Since the reaction involves liberation of CO2, it is inhibited by high pressures. |
700 C
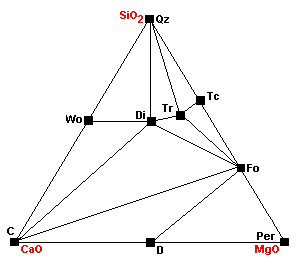 | At about 740 C, magnesite breaks down to periclase (MgO): Mag = Per + CO2 |
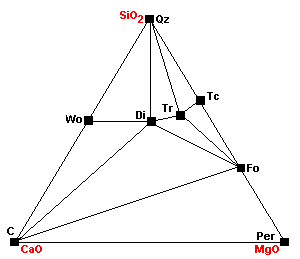 | At about 780 C, dolomite breaks down: Dol = Cal + Per + CO2 |
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 Sept 1997, Last Update