Biopyriboles
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Building double chain and sheet silicates from single chain silicates is mostly a matter of linking chains side by side. Hypersthene, a pyroxene [(Mg, Fe)SiO3], Anthophyllite, an amphibole [(Mg,Fe)7Si8O22(OH)2] and Biotite mica [K(Mg,Fe)2(AlSi3O10)(F,OH)2] all consist of layers of iron and magnesium hydroxides or oxides sandwiched between layers of silica tetrahedra. Many of the same atomic structures occur in pyroxenes, amphiboles and biotite. The connection was first noted by Albert Johannsen of the University of Chicago, who coined the term in 1911.
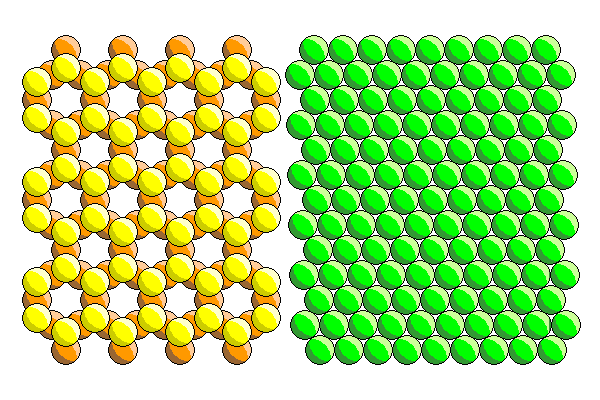
At first glance, it looks like fitting a silica sheet and a ferromagnesian sheet together should be a piece of cake. After all, the atoms in both sheets are in hexagonal arrangements and the anions in both sheets are oxygen or hydroxyl (OH). So the sheets should mesh nicely. (If the ferromagnesian sheet contains only magnesium, it is the mineral brucite. For that reason, the ferromagnesian sheet is often called the brucite sheet. It is also often called the trioctahedral sheet.)
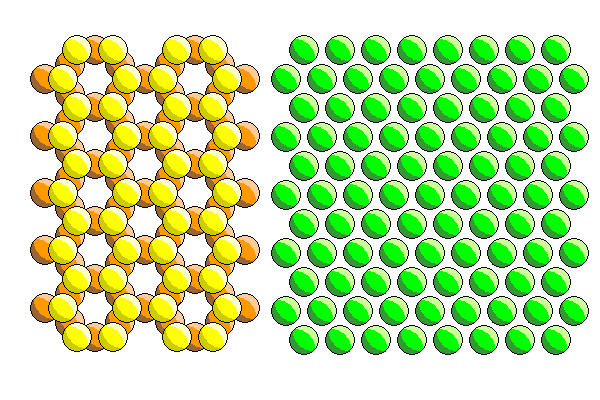
The problem is, it's not simply a matter of just putting a cation into a tetrahedral cavity and putting another anion on top. The apical atoms in the tetrahedral silica sheet (yellow) fit into the ferromagnesian sheet. The apical tetrahedral anions are shared with the ferromagnesian sheet. The adjacent apical tetrahedral atoms don't touch. And there is no arrangement of atoms in a close-packed hexagonal sheet that matches that spacing. The only way it will work is if the anions in the ferromagnesian sheet aren't in contact, with the same spacing as the apical anions in the silica sheet.
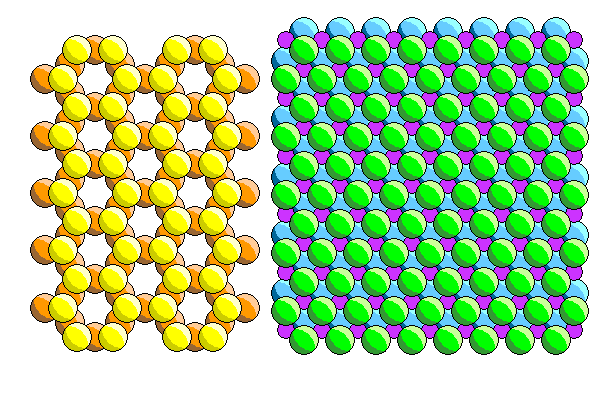
The ferromagnesian sheet looks like that above at right. The cations (purple) fit into octahedral interstices between hexagonal, but not close-packed, layers of anions (green for the upper layer, blue for the lower. Spacings in both sheets are not exactly what they would be in isolation, and neither sheet is especially happy about it.
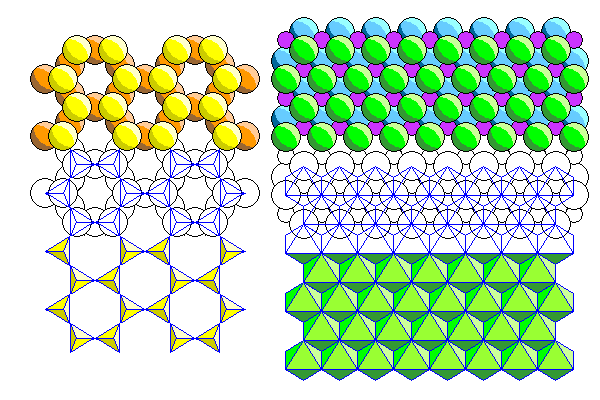
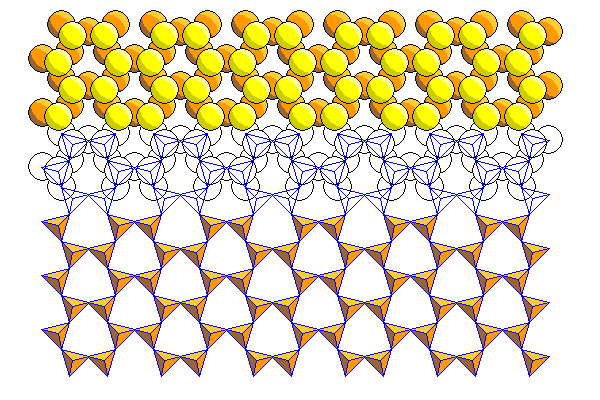
Above are the silica sheet (left) and ferromagnesian sheet (right) showing a pictorial representation above and a polyhedral representation below. The relationship between the two models is shown in the middle where both overlap.
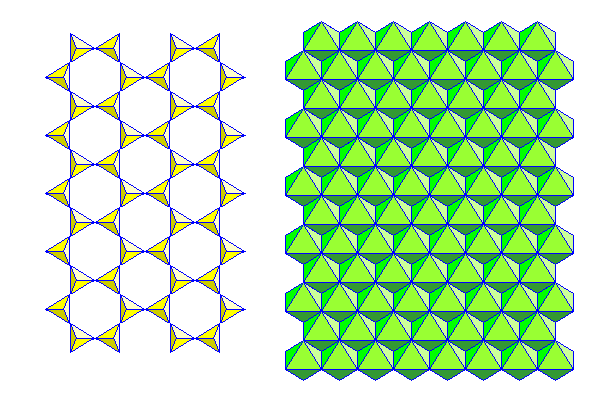
Above are the silica and ferromagnesian sheets in polyhedral form.
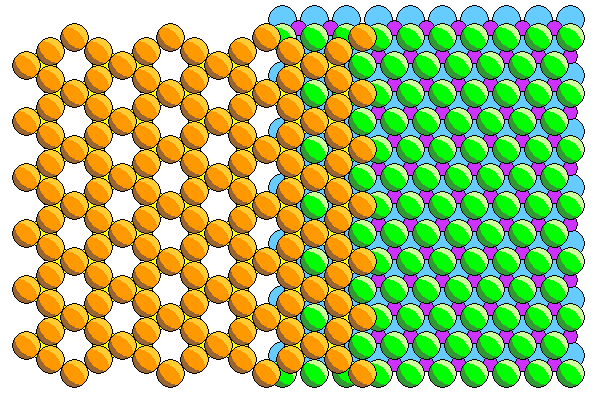
The mating of the silica and ferromagnesian sheets is shown above. Here we see the back of the tetrahedral silica sheet with the apical atoms (mostly hidden) in yellow. The apical atoms share two thirds of the sites in the ferromagnesian layer. The ferromagnesian atoms are not in the holes of the silica sheet but in the next laver below.
We can see that the hexagons in the silica sheet are oriented perpendicular to those in the ferromagnesian sheet. In the silica sheet are vertical rows of adjacent atoms, whereas there are horizontal rows in the ferromagnesian sheet.
Revenge of the Brucite Sheet
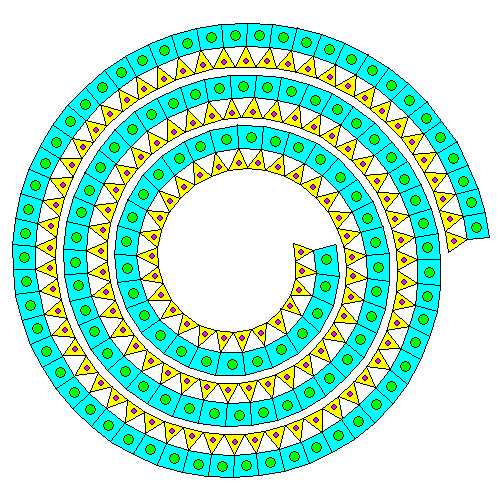
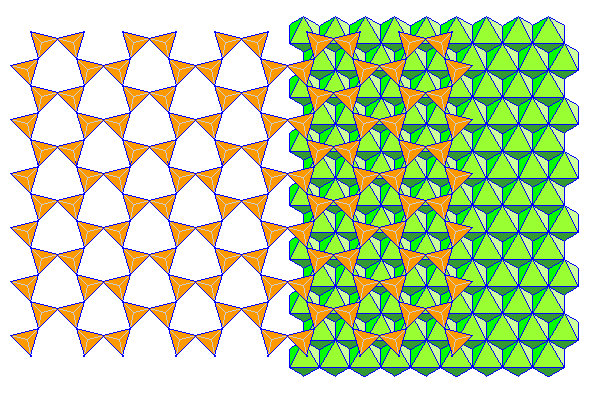
Neither the silica sheet nor the brucite sheet especially like being strained. If there's only a silica sheet on one side, the mismatch causes the pair of sheets to curl into spirals and tubes. Thus, even though it's a phyllosilicate, the mineral ends up forming thin fibers. This is the serpentine form of asbestos. In the diagram above, silicon atoms are orange and silica tetrahedra are yellow. Magnesium atoms are blue and magnesium octahedra are green.
"Go ahead, deform my lattice. Just for that, I'll give the humans mesothelioma. Buwahahahaha." Actually, it appears that serpentine asbestos dissolves fairly quickly in the body and that amphibole asbestos is the real villain. The reason we regulate all asbestos is the types can't be distinguished easily and it's very likely that serpentine asbestos will also contain amphibole asbestos.
The silica sheet also deforms. A silica sheet in isolation would have the open hexagonal structure shown above because the anions would repel each other. But if there are nearby cations, they can overcome some of the anion repulsion. If the tetrahedra rotate so they no longer form regular hexagonal rings, the spacing of the apical anions becomes smaller.
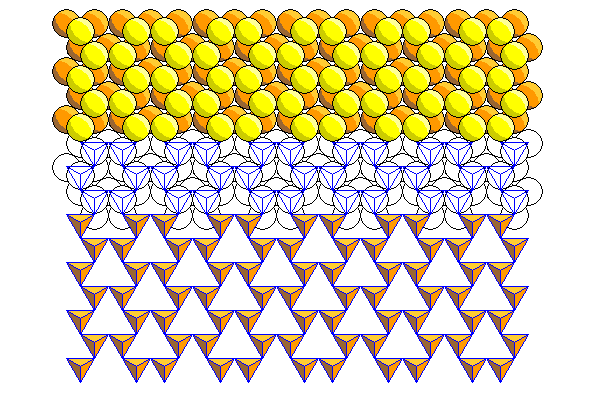
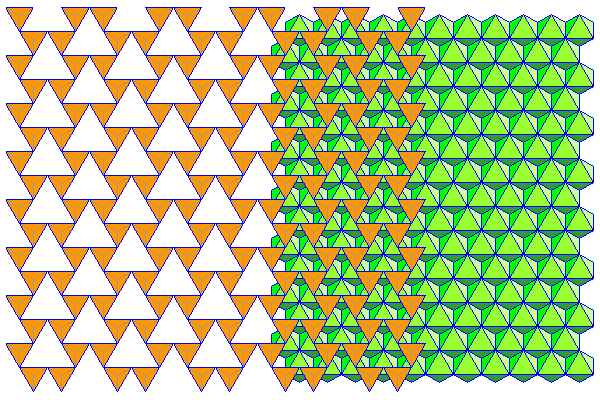
In the extreme case shown here, the tetrahedra have rotated alternately clockwise and counterclockwise by 30 degrees. The apical tetrahedra actually touch. This sheet matches the spacing in a close-packed sheet perfectly. All the yellow atoms would be shared with the close packed sheet, occupying two thirds of its sites, and the remaining atoms would fill the holes in between. Usually there isn't enough cation attraction to result in this sort of deformation.
In most cases the rotation is not so extreme. Here the tetrahedra have rotated 15 degrees. This actually represents the configuration in biopyriboles more accurately than the ideal hexagonal sheet usually presented in textbooks.
Because Al ions are small, the dioctahedral gibbsite sheet is nearly close packed and the silica sheets are almost in the triangular arrangement shown above (the tetrahedra are rotated 27 degrees from the ideal hexagonal arrangement rather than 30). Magnesium ions are bigger and the arrangement is more like the second configuration above. The tetrahedra are rotated 12 degrees from the ideal hexagonal configuration rather than the 15 depicted above. Ions much bigger than magnesium cannot form sheets that register with the silica sheets. Ferrous (+2) iron is pushing it.
Here we have been dealing with sheet silicates. In amphiboles and pyroxenes we have strips of silica tetrahedra and ferromagnesian octahedra. Since the octahedra on the edges of the strips are not bound to the silica strips above and below, but are bound to silica strips on the side, the outer octahedra are often quite distorted and silica chains with rotated octahedra are common.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 08 April, 2005, Last Update