Cancrinite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Nepheline and Leucite are called feldspathoids because they have chemical compositions akin to the feldspars but form in silica poor rocks where there is insufficient silica to form feldspars (and of course, they never occur with quartz). Cancrinite might be called a "scapolitoid" since, like scapolite, it has a feldspar-like composition with additional anions.
Many references describe cancrinite as consisting of silica "cages" enclosing other anions and cations, but that's a poor description of the structure. The "cages" don't reflect the actual geometry of the silica framework very well, nor do they nest together as sometimes shown. The structure actually consists of tubes of silica tetrahedra surrounded by rings of tetrahedra as shown below.
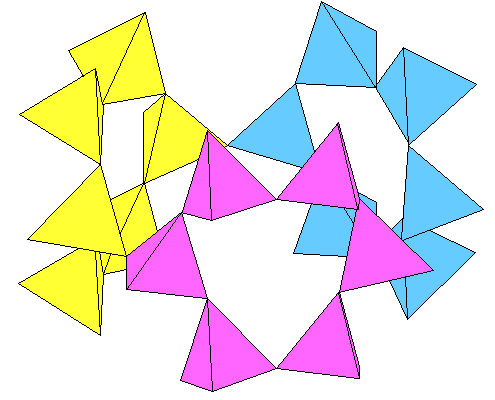
The silica tubes are 12-sided and link at the outside tips of the tetrahedra. Three adjoining rings enclose hexagonal tubes, as shown below. Silica and AlO4 tetrahedra are green, Sodium and calcium ions are yellow, carbonate ions are shown with carbon gray and oxygen blue (lighter in the background) and OH as aqua.
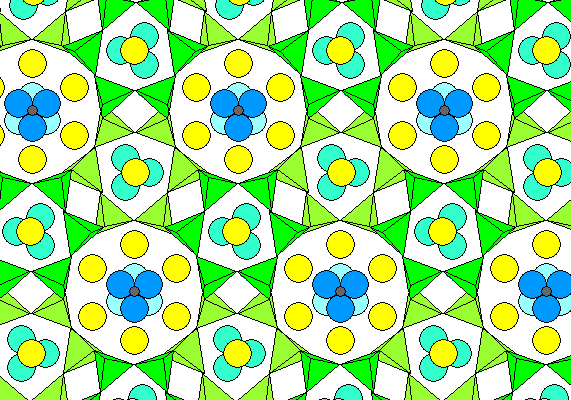
The ideal formula is Na6Ca2[(CO3)|2Al6Si6O24]2H2O. As is clear from the diagram, there is broad scope for substitution of anions and cations.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update