Carnallite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Carnallite has the formula KMgCl36(H2O) and occurs as an evaporite from hypersaline brines. The name hints that it might have been named for its common pink or flesh color, but in fact it is named for mining engineer Rudolf von Carnall (1804-1874).
One site describes the structure of carnallite as very difficult to visualize, and it is a challenge, made far worse than it needs to be by the refusal of many mineral structure data bases to make effective use of coordination polyhedra. (Probably because it's easy to plot atoms straight out of an X-ray structure data file, but defining coordination polyhedra requires a lot more work.) Carnallite is described as a "double chloride" but that's not entirely accurate. The magnesium atoms are enclosed in octahedral cages of water molecules and are not directly in contact with chlorine atoms at all. The potassium atoms are octahedrally coordinated to chlorine atoms. four octahedra are linked corner to corner to enclose a square, and the octahedra at opposite ends of a square are joined face to face with the octahedra of adjoining squares.
Below is a view of the carnallite structure viewed down the a-axis, showing only the KCl octahedra. The b-axis extends horizontally and the c axis vertically. The colors indicate distance above the plane of the diagram, with yellow being closest to the viewer and blue farthest away.
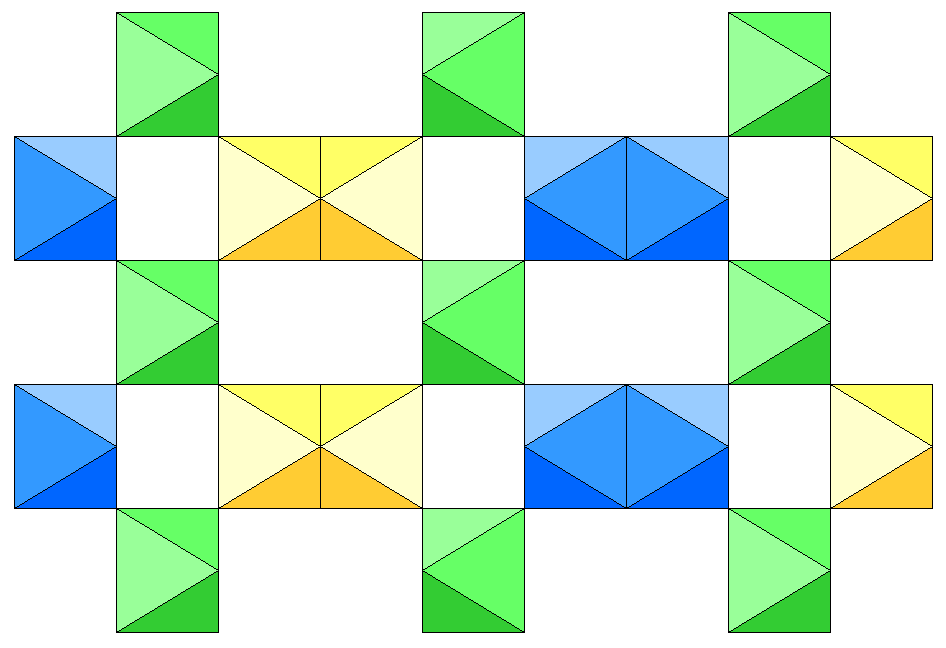
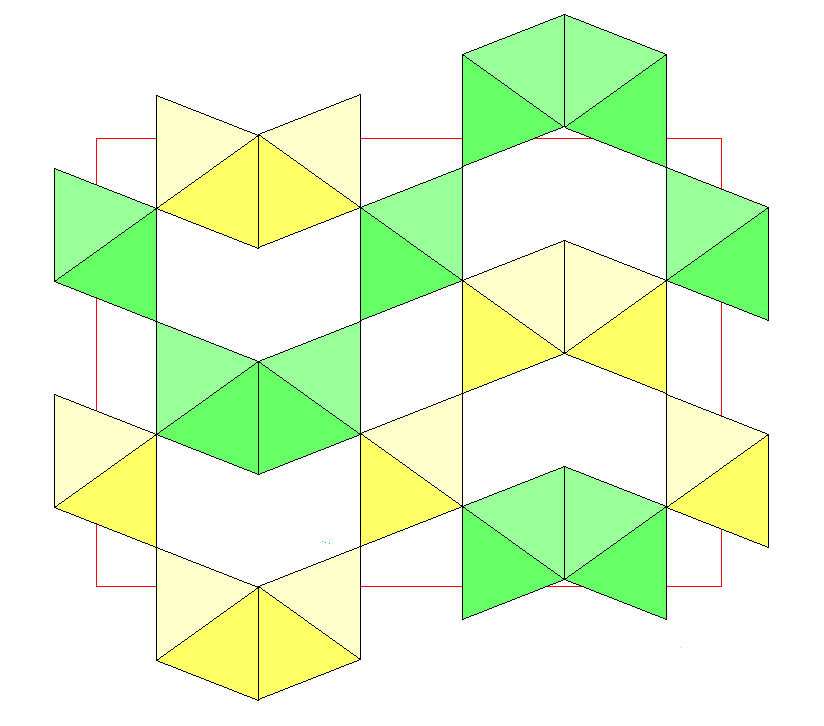
Below is a view along the c-axis showing only the K-Cl octahedra. The a-axis is vertical and the b-axis is horizontal. The colors again indicate distance above the plane of the diagram, with yellow being closest to the viewer and blue farthest away. However, note that a given octahedron may have different colors in the two diagrams. At top center, a blue octahedron is flanked by two yellow ones. This is one of the square units, with the frontmost octahedron not shown. The top corners of the blue octahedron are linked to the bottom corners of the yellow ones. The green octahedra are above the blue octahedron and are not joined to it, even though the vertices of the green and blue octahedra line up. Note that the face to face joining of octahedra creates a wavy structure.
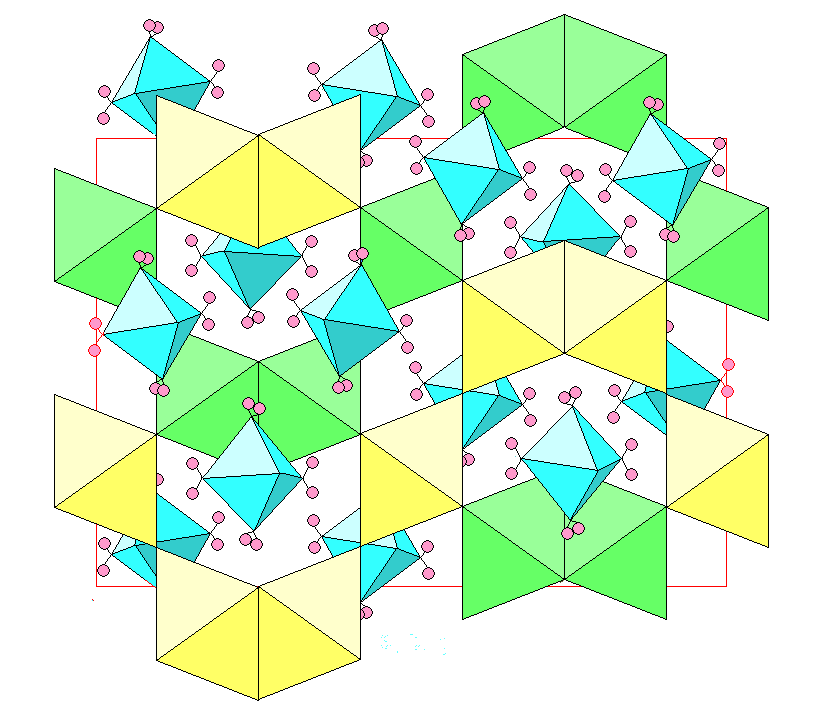
The view below shows the magnesium-water octahedra in light blue. An oxygen atom is at each vertex and hydrogen atoms are pink. The unit cell is shown in red.
Below is an oblique view showing only K-Cl octahedra. Yellow, green, blue and gray represent increasing distance from the plane of the figure.
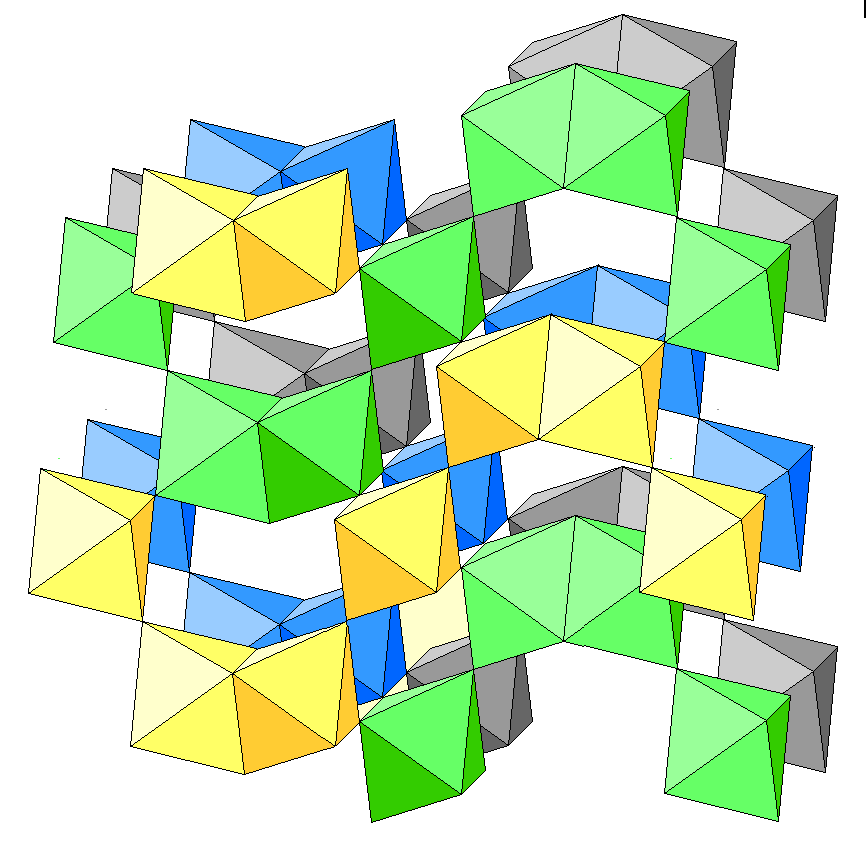
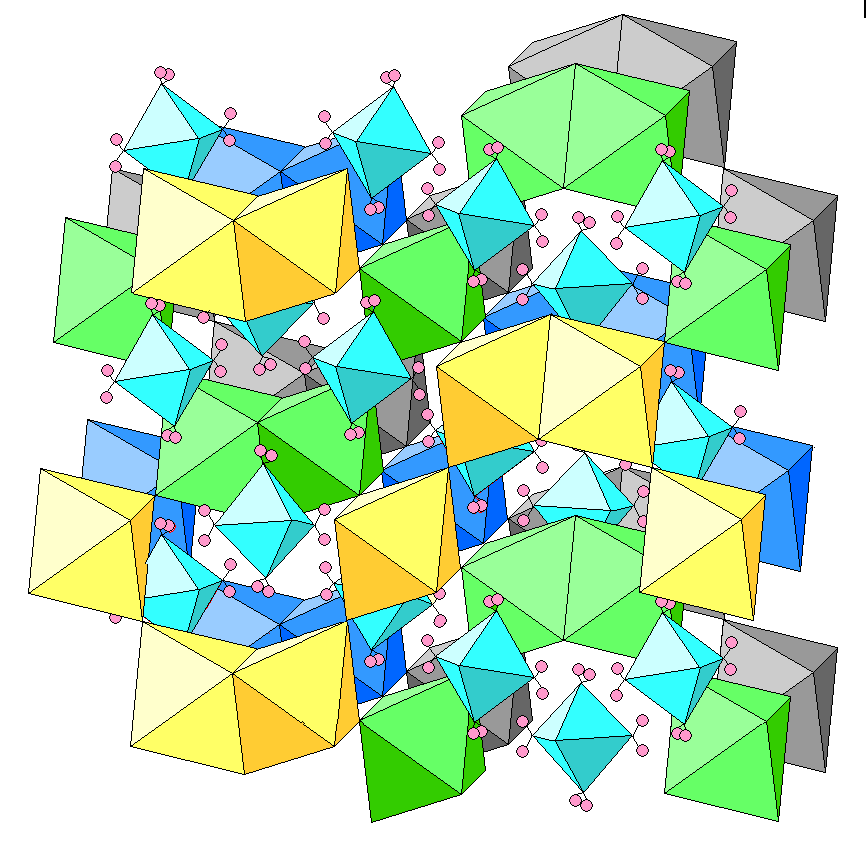
Below is an oblique view showing K-Cl octahedra, with yellow, green, blue and gray represent increasing distance from the plane of the figure. The magnesium-water octahedra are in light blue. An oxygen atom is at each vertex and hydrogen atoms are pink.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 15 March 2011, Last Update