Chalcanthite
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Chalcanthite, CuSO45(H2O), earns an "honorable mention" as a musheral because it doesn't quite have as many water molecules as other atoms. It has six atoms in its formula versus five water molecules. Nonetheless it's pretty watery. It gains and loses water easily and has a tendency to disintegrate in collections unless carefully stored. Siderotil (Cu,Fe+2)SO45(H2O), Pentahydrite MgSO45(H2O) and Jokokuite MnSO45(H2O) are essentially isostructural.
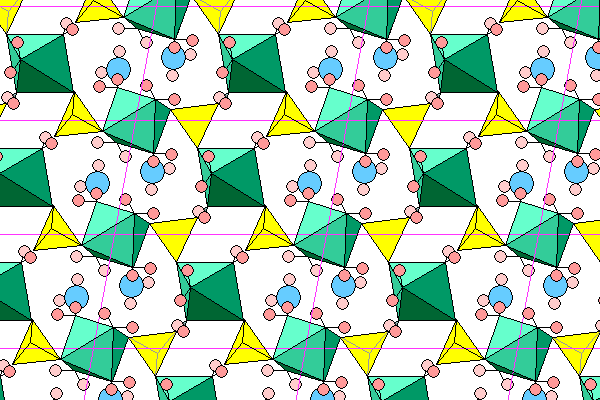
Chalcanthite is triclinic. This view looks down the c axis at the a-b plane. The structure consists of octahedra (green) surrounding the copper atoms. The octahedra share oxygens at either end with sulfate tetrahedra (yellow). The other four vertices are water molecules with hydrogens in pink (dark in foreground, light behind). There are also two independent water molecules in each unit cell, with their oxygens shown in blue. The octahedra and sulfate tetrahedra form zigzag chains that also undulate above and below the plane of the diagram.
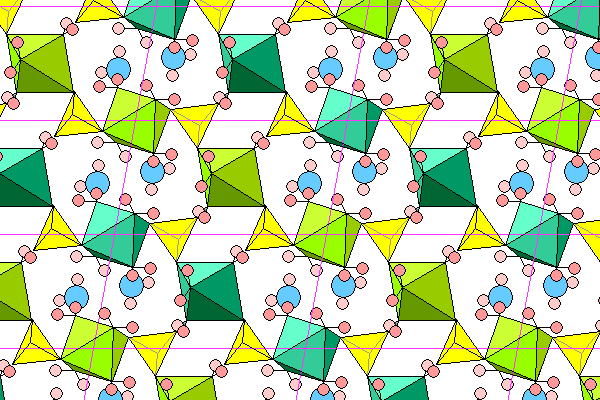
This view is the same as above, but individual chains are alternately colored.
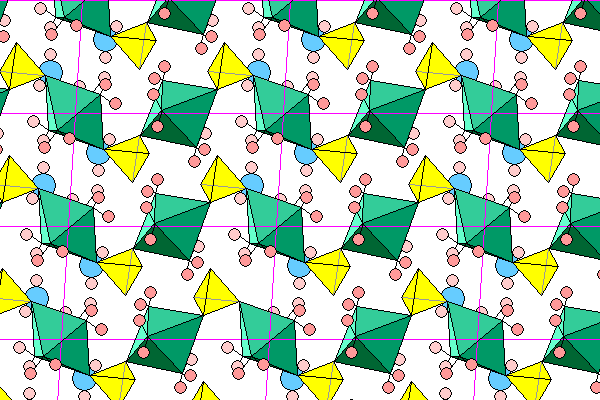
This view looks down the a axis, with the c axis across the diagram and the b axis nearly vertical. Although the chains look continuous, that's merely a perspective effect due to overlap. In each unit cell, the chains extend from left front to right rear. The left and middle octahedra are joined, but the right rear octahedron is concealed by a new chain entering the picture.
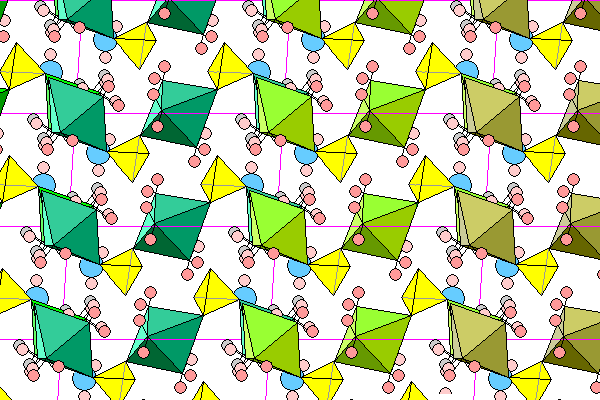
This view shows successive chains in different colors, with front and rear corner octahedra slightly offset to show the overlap. Rearmost hydrogens are light gray. Chains extend from left front to right rear.
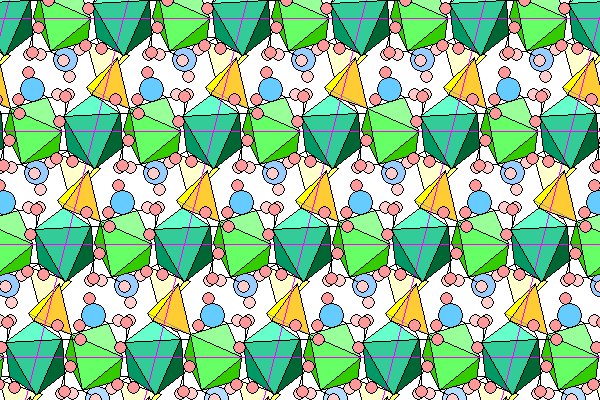
This view looks along the b axis. Again, although the chains look continuous, that's merely a perspective effect due to overlap. In each unit cell, the chains extend from left front to right rear.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 28 March 2011, Last Update