Chesterite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences,
Chesterite isn't a conspicuous mineral or very abundant. It's probably more abundant than we think simply because people don't look for it very often. But it's a very important mineral because it was the first mixed-chain silicate ever discovered (1978). Double and triple silica chains alternate. In these figures, silica tetrahedra are purple, Mg-O octahedra are green and iron polyhedra are yellow.
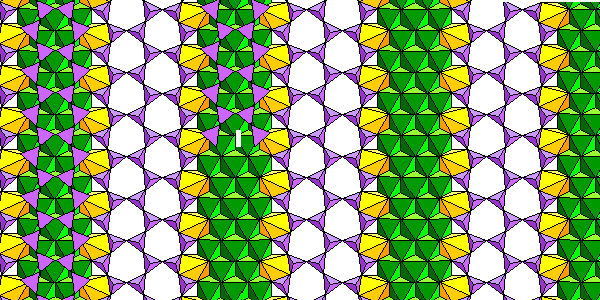
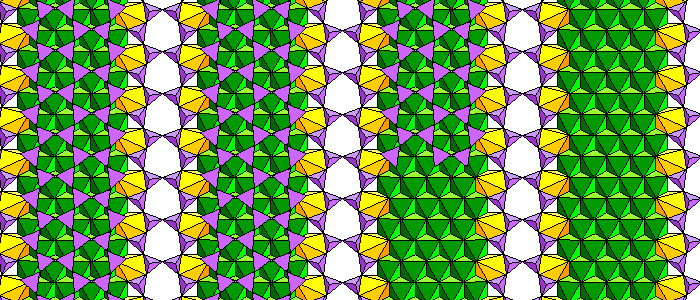
The structure is actually pretty simple. Think of the triple chains as a stretch-limo version of an amphibole, with the silica and cation chains split down the middle and an extra chain added. Triple cation chain layers alternate with layers of more or less typical amphibole cation chains. Within the silica layers, double chains and triple chains alternate.
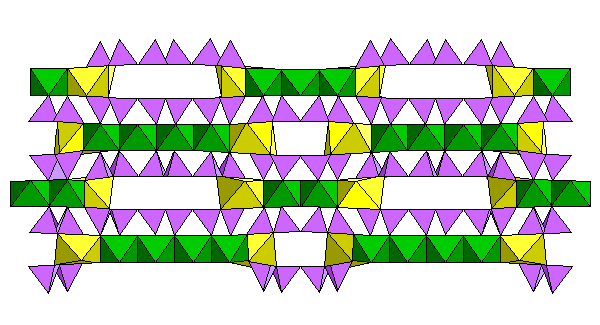
Structure viewed end-on. The wide voids between the triple strips suggest there are probably space-filling atoms or water molecules in there helping to bind the layers together and prevent collapse. There are probably many stacking and ordering variations possible.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update