Chloraluminite
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Winston Churchill once famously described Russia as "a riddle, wrapped in a mystery, inside an enigma." Chloraluminite, AlCl3*(H2O), can be described as an aluminum atom wrapped in a water octahedron inside a chlorine icosahedron.
"God is in the details" (often attributed to architect Mies van der Rohe) or as often parodied, "The devil is in the details." Unfortunately, most available depictions of chloraluminite show only a single unit cell and only the water-aluminum octahedra, making it all but impossible to see the larger structure.
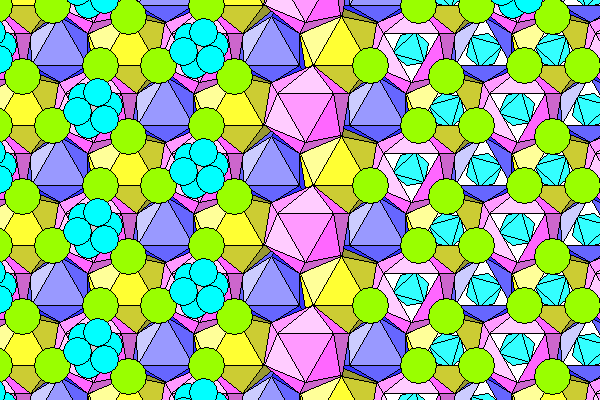
The drawing below shows two layers of water-aluminum octahedra (blue) and chlorine atoms (green). Foreground objects are in darker colors. The aluminum atoms (gray) are at the centers of the water octahedra. The chlorine atoms are in twelve-fold coordination, forming somewhat distorted icosahedra. In the view shown here, looking down the c axis, each icosahedron is made up of four triangles of chlorine atoms. With increasing depth into the diagram, the layers consist of a closely spaced triangle (which we can label A), a more widely spaced triangle (B), another widely spaced triangle inverted 180 degrees from B (call it C) and finally a closely spaced triangle (D) oriented 180 degrees differently from A. The D layer of one icosahedron is the A layer of the next one down, and columns of icosahedra are staggered vertically, so the A layer of one is coplanar with the B or C layer of the next.
On the left side of the diagram below, we see two layers of chlorine atoms enclosing water-aluminum octahedra. Only the oxygen atoms are shown. The lighter colored (background) octahedra are overlain by an A layer of darker atoms and a B layer of lighter ones. The darker (nearer) octahedra are surrounded by a B layer (dark) and a C layer (light). The A layer of the background icosahedra is coplanar with the B layer of the foreground icosahedra. In the middle we see only the background octahedra and their C layer, and on the right just the skeletal icosahedra. Purty, ain't it? I've had Easter baskets with fewer colors.
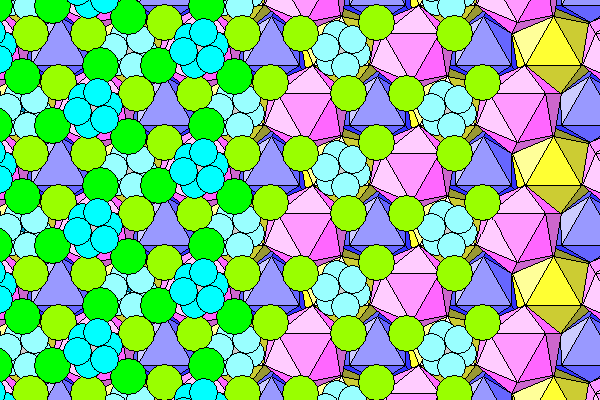
Here we see just the coordination icosahedra. On the left are the layers seen above, with pink in the foreground, yellow behind, then dark blue exposed behind that. On the right, the holes are filled by the next icosahedra above (light blue).
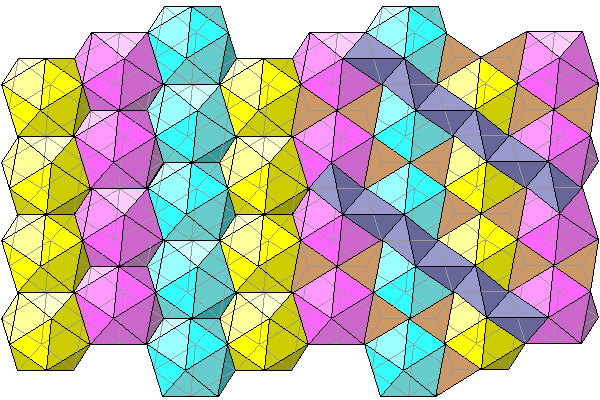
The icosahedra don't fill space but have irregular tetrahedral voids between them. Only the vertically adjacent icosahedra share faces; the neighboring columns share edges. On the right two sets of tetrahedra are shown. The gray set extends beneath the light blue icosahedra. The brown set is shown filling the gaps between the pink and light blue icosahedra, with the top faces of the tetrahedra added..
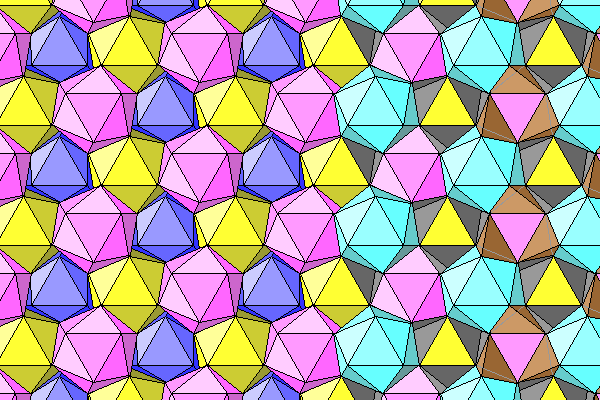
Here we see the coordination icosahedra, and on the right the top faces removed to reveal the inner water-aluminum octahedra.
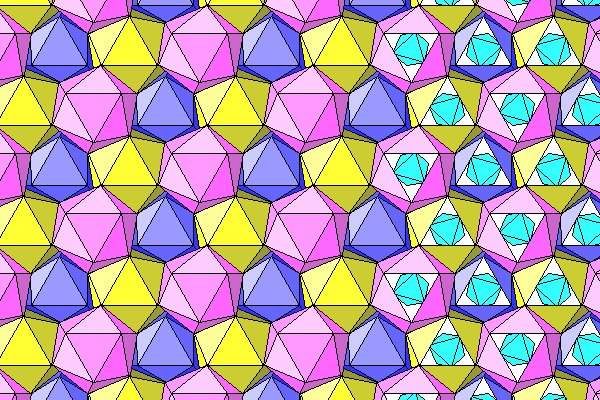
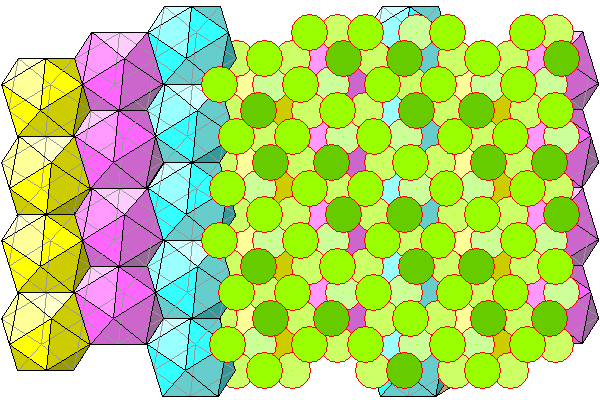
Same diagram as above but showing chlorine atoms and water on the left. The chlorine atoms are lighter because they indicate the B layer of the pink icosahedra and the A layer of the yellow ones.
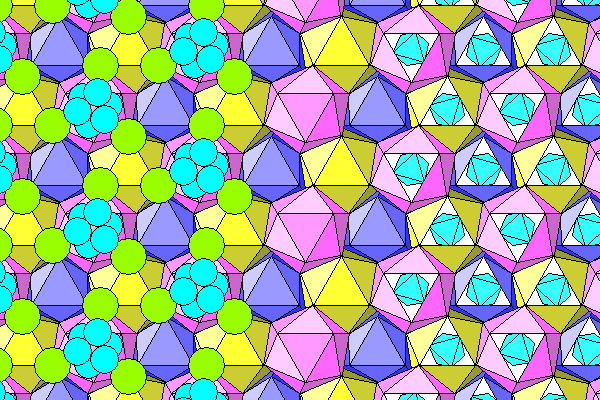
And finally a diagram showing chlorine and water on the left, and chlorine atoms and water-aluminum octahedra on the right.
Below are a series of diagrams viewed perpendicular to the c-axis. The plane of the diagram is the long diagonal of a unit cell rhombus. The first diagram simply shows the chlorine coordination polyhedra. On the right, the tetrahedral voids are indicated in gray and brown.
Below, we see columns of icosahedra on the left and chlorine atoms on the right. All chlorines are shown whether on the front or rear of the icosahedra.
Below is a diagram showing only the foreground chlorines with darker atoms closest to the viewer. The relationship between the chlorine atoms and the tetrahedral voids is clearly shown.
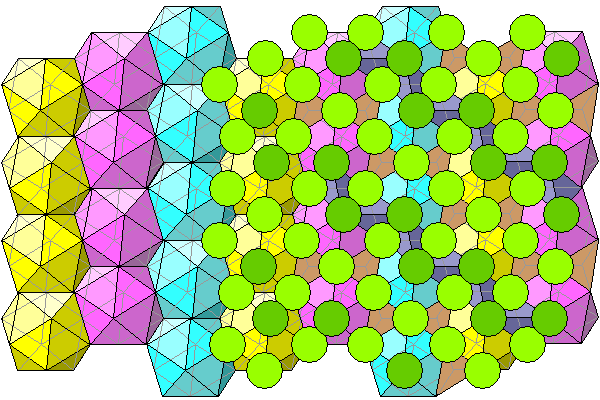
Below, the right side of the diagram shows all chlorines without polyhedra.
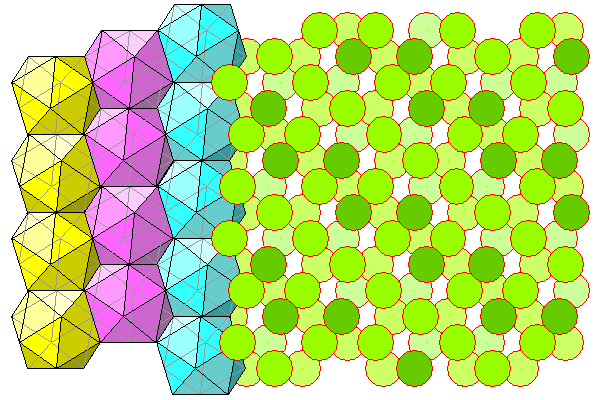
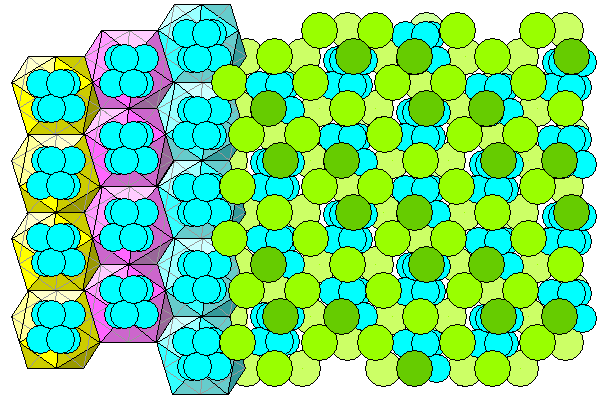
Finally, a view of the aluminum-water octahedra and the enclosing chlorine icosahedra (hydrogen atoms not shown).
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 8 April 2011, Last Update