Causes of Color: Incandescence and Simple Excitations
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
These are probably the conceptually simplest causes of color. Incandescence is simply the emission of electromagnetic radiation by all objects, but it only becomes visible at fairly high temperatures. Simple excitations refer to the emission of light by single atoms or molecules under stimulation by an energy source.
Incandescence
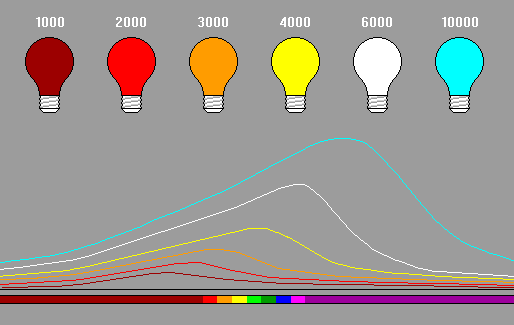 |
Black body radiation paradoxically refers to the light given off by hot objects. The term "black body" is used because the body is not reflecting light from any other source or emitting radiation by any other process. |
The figure above corrects a widespread distortion in many references. Diagrams often show the black-body curves for different temperatures as overlapping, to avoid having an exaggerated vertical scale. In reality, black body curves for different temperatures never overlap. The curve for a given temperature is above the curve for a lower temperature at every wavelength, and below the curve for a higher temperature at every wavelength.
The colors for black-body radiation have a restricted spectrum. The color starts out dim red when incandescence first appears, then becomes brighter red, orange, yellw, then white. We never see green because by the time the radiation peak is in the green (like the sun at 5700 K), there is so much emission across the spectrum that the color is white. However, when the peak shifts into the ultraviolet, the emission of blue light is far greater than the red wavelengths, so the object can appear blue-white.
One scientifically powerful application of black-body radiation is that color is related to temperature and does not depend on the reason the object is hot. So if you see Antares on a summer evening, and a porch light of similar brightness and color, the strar and the light are of equal temperature (about 2500 K). A forest fire, a lava flow, a vat of molten metal and a nuclear fireball of the same color are all at the same temperature.
Another exceedingly powerful application of black-body radiation is that the radiation emission per unit angular area is exactly the same for any given temperature. For example, the Star Sirius is blue-white. It has a faint companion, also blue-white, and therefore at the same temperature. Since they're the same distance from us, the difference in brightness must mean they differ in size. When we refer to stars as giants and dwarfs, they are literally giants and dwarfs in size. (Since Sirius is sometimes called the "Dog Star," its tiny companion is sometimes called "the Pup.")
Old-time star catalogs sometimes reported fanciful star colors like green or violet. Green is probably a contrast effect from a faint white or yellow star being seen next to a bright reddish star. It's also probably due to the eye being most sensitive to green light. Surprisingly, some stars can be purplish, a result of a very red star having carbon molecules that absorb green and blue light.
 |
Slag being skimmed off a crucible in the smelter at Sudbury,
Ontario. The slag emits light because it's hot and the specific
awvelengths are directly related to temperature. A star emitting
that same color is the same temperature. Because it's a long exposure, drops of spattered slag leave streaks. Fun fact: if one of those drops is the same color and brightness as, say, Betelgeuse, they are the same angular size. |
 |
A slag dumping at night. Note how the color changes to red as the slag cools |
 |
Flames emit nearly black body radiation. Incomplete combustion creates tiny soot particles which are heated to incandescence. The smoke is suspended soot particles that have cooled below incandescence. |
Gas Excitations
Stellar Spectra
Atoms in gases emit light when an outer electron is stimulated by energy to a higher energy level. For any given atom, only certain energy levels are possible. Eventually the electron drops back down to its original, or ground state. When it does, the energy is released as light.
This means that elements can be identified by the specific wavelegths they emit or absorb. The Sun emits a continuous spectrum,with all wavelengths present, but as the light passes through a thin outer layer some light is absorbed, If we spread sunlight into a spectrum, those absorbed wavelengths show up as thin, dark lines. The absorbed light is re-emitted, of course, but in all directions, not just radially, and therefore there's not enough emission to cancel out the absorption. During a total eclipse of the sun, as the last visible disk of the Sun is covered, the thin layer, called the chromosphere, shows up as a spectrum of bright lines called the flash spectrum. Although it is thin, the chromosphere, in a sense, is the most important layer in the Sun, or any star, because it's the Absorption lines in the spectrum produced by the chromosphere taht tell us the composition of the stars.
When challenged to give an example of something we could never know, the philosopher Auguste Comte suggested we would remain forever ignorant of the composition of the stars. A couple of decades later, spectroscopy was discovered. That didn't take long.
Flame Tests and Fireworks
Atoms can also be excited by subjecting them to heat, for example in flames. Before instrumental techniques became cheap and ubiquitous, one technique for detecting elements was by putting a sample on a non-reactive wire (platinum if the budget allowed, various other alloys if it didn't). The sample was then held in a Bunsen Brurner flame. Sodium gave a yellow flame, copper and barium gave green, strontium and lithium gave red. In fact, most everything gives a yellow flame because sodium is so common and can easily contaminate samples and apparatus. In addition, anything that burns with a typical flame will probably emit black-body radiation which will be very hard to distinguish from a sodium flame.
Not only is sodium a widespread contaminant in flame tests, but if there's anything that causes incomplete combustion, there will also be a yellow incandescent flame
Pyrotechnics, like flares and fireworks, rely on excitation of selected atoms. Fireworks typically include magnesium, which incandesces brilliant white, as well as colorants like strontium, sodium, barium and copper.
 |
White is produced by extremely hot burning magnesium (incandescence) but colors are due to emissions by copper, strontium, and other atoms. |
Neon lights and Auroras
 |
This is good street lighting. The light is illuminating the ground, not the sky. The yellow is due to sodium vapor lighting and the greenish is mercury vapor. |
 |
Las Vegas was described by Rita Rudner as "The only place in the world where the sun goes down, and it gets brighter." In this 2005 photo, the colors are mostly neon and other gases. Nowadays much of this lighting has been superseded by LED's (light-emitting diodes). |
A neon light is a sealed glass tube with low pressure neon inside. An electric arc excites the atoms inside, causring them to emit light. Neon is a low-cost by-product of liquefying air. The tern "neon light" has become generic for any gas tube excited by electrical discharge, regardless of the gases used. The ubiquitous flourescent lights used for illumination are similar. They generally are filled with mercury vapor, which emits mostly ultraviolet, and the interior of the tube is coated with a fluorescent compound that glows when struck by ultraviolet.
Auroras have been called "nature's neon lights," and emit light because gases are struck by charged particles. In this case, the charged particles come fron the Sun and are funneled toward the magnetic poles by the earth's magnetic field. There is generally a steady aurora in a circle surrounding the magnetic poles, called the auroral oval. When especially large outbursts from the sun strike the earth, the auroral oval can spread outward to Europe and the United States. On very rare occasions, auroras can extend into the tropics.
The predominant colors of auroras are red and green. Red is emitted by oxygen at high altitudes, green by oxygen at lower altitudes. Nitrogen molecules sometimes (rarely) emit blue light. Other colors sometimes appear due to mixtures of these colors.
 |
Aurora over the Southern Ocean, seen from the Space Station. Spacecraft views enable us to see the three dimensional forma of auroras. When auroras look like curtains, they are curtains. The red auroras are above the green auroras |
Sprites
Sprites are a good example of a phenomenon remaining dubious for a long time before new technology made it possible to study it. Sprites were a nickname that high-altitude pilots applied to brief red or blue jets above thunderstorms. The observers were well trained and disciplined, and were obviously seeing something, but what? For a long time, a handful of ambiguous photographs were the only evidence. You needed to have a camera ready at exactly the right instant.
Once good digital photography became possible, the situation changed radically. Sprites have been phoographed from the ground, from aircraft, and even from space. They only last milliseconds. We still don't know exactly what they are or how they form, but their existence is beyond doubt.
|
|
The airglow lyer appears as a sharp yellow-green band, with stars visble between it and the true horizon. At right is a cloud brilliantly illuminated by lightning, and above it are red sprites, photographed from the Space Station. The yellow splotches are cities illuminated by sodium vapor lights. Pretty much everything in this picture is some form of gas excitation. |
|
|
Thanks to high-speed dgital photography, photographs of sprites are becoming commonplace. They appear above large thunderstorms and last milliseconds. |
Chemiluminescence and Airglow
Chemiluminescence is light given off by chemical reactions. Fireflies, certain fungi and various marine organisms were the most widespread natural sources of chemiluminescence. Artificial sources are now commonplace in the form of glow sticks that emit light when two chemicals are mixed. The chemicals are kept separate by a thin barrier that is broken by bending the tube.
 |
The business end of a firefly. Chemiluminescence in action. In biology it's called bioluminescence, but it's still chemical in nature. |
One interesting natural form of chemiluminescence is airglow. Thanks to space travel and high quality color digital imagery, photographs of airglow are widespread. Airglow should not be confused with the aurora.
When viewed from orbit, a thin luminous layer appears in the atmosphere at about 80-90 kilometers altitude. It's generally very sharp along the limb of the earth because our line of sight passes through a large thickness of the luminous layer. The color is mostly due to oxygen, and particularly the reversal of the reactions that create ozone. During the day, solar ultraviolet excites oxygen atoms and causes them to react to form ozone, but at night, without ultraviolet stimulation, the ozone breaks down into oxygen atoms and emits light. The airglow layer is global and means the night sky is never wholly dark. Between airglow, light scattered from dust in the plane of the Solar System, and starlight, it can be possible to read a newspaper headline at night. Not fine type, but the headlines.
|
|
The greenish layer is not the edge of the earth, because stars are visible beneath it, but the airglow layer. It stands out prominently because our line of sight passed through a great thickness of it. Note the red sprites at upper right. |
 |
Airglow happens during the day, in fact more intensely because of the high levels of ultraviolet, but it's invisible against the bright sky. It can be seen at twilight as a persistent glow well after sunset. Greenish twilight colors are enhanced by airglow. |
Vibrations and rotations
Vibrations and rotations primarily affect molecules in the infrared. The absorption of infrared radiation by carbon dioxide, methane and water vapor is the principal cause of the "greenhouse effect,"
Vibrations of water molecules are mostly in the infrared but trail off into the visible spectrum. They absorb some red light, but not blue, so water preferentially transmits blue light. The absorption is very slight, so absorption only appears in masses of water meters thick or larger.
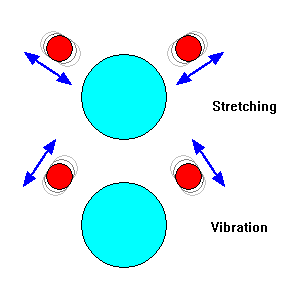 |
Vibration modes of water molecules. Bonds can stretch, or bond angles can increase and decrease. |
 |
Ice and water really are blue. Vibrations of water molecules absorb mostly infrared, but a tiny amount slops into the visible spectrum, so substantial thicknesses of ice and water actually are blue in color. |
 |
The Hubbard Glacier in Alaska. No blue sky, in fact it's foggy and dreary. Yet the ice is blue. |
 |
 |
 |
The water here is only about two meters deep but obviously blue. Most biological blues, like the fish, are due to scattering or interference. |
 |
Big Spring in upper Michigan is crystal clear and up to ten meters deep. And blue. |
Water really is blue, not merely reflecting light from the sky or scattering light, though those play a role. Blue ice really is blue, not merely scattering light from air bubbles or dust particles.
 |
Uranus (left) Neptune (Right) |
 |
Another gas that similarly absorbs infrared but also absorbs somewhat at the red end of the visible specrtum is methane. Methane is why Uranus and Neptune appear blue.
Combustion results in some interesting effects. Incomplete combustion creates carbon particles, which glow yellow due to black body radiation and leave behind a deposit of soot. Carbon monoxide is also being produced. No, the yellow of a candle flame does not indicate a hazard. A lot of yellow in a gas furnace may very well indicate a hazard, however.
If combustion is complete, though, something interesting and unexpected happens. Carbon chemicals break down to produce C-C and C-H molecules. These have myriad spectral bands in the visible spectrum, mostly in the blue and green. These emissions, called Swan bands, cause the blue color of a kitchen burner flame. They can also result, startlingly, in blue and green flames in a campfire. These colors are often, erroneously, attributed to copper.
 |
Gas flames burn methane and create C-C and C-H molecules, which are excited to produce Swan Band colors, notably the typical blue flame. |
C-C and C-H are emitted by comets, and are responsible for the bright green color of many comets. In cool, carbon rich red stars, the atmosphere may be rich in C-C and C-H. Here, the molecules absorb blue and green light, so the resulting color consists mostly of red and violet and the star can appear magenta.
 |
Not only do Swan bands show up in some flames, but surprisingly enough in the colors of comets. Comets emit a lot of green and blue light and much of it is Swan Band emission. This is Comet Holmes, which had been plodding uneventfully through the inner Solar System since its discovery in 1892, then suddenly brightened by a factor of half a million in 2007 to become visible to the unaided eye |
Kurt Nassau and the Fifteen Causes of Color
Atoms and Light
Causes of Color: Energy Bands
Causes of Color: Ligand Fields
Causes of Color: Molecular Orbitals and
Charge Transfer
Causes of Color: Physical Optics
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update