Causes of Color: Ligand Fields
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
 |
At left are the orbital configurations for the elements Nitrogen (7)
through Neon (10). There is an inner 1s orbital containing two
electrons, then an outer 2s orbital with two electrons, and three outer
p orbitals with opposing lobes, each with one or two electrons. After Neon, atoms from Sodium (11) to Argon (18) add a third shell, again consisting of a 3s orbital and three 3p orbitals. |
 |
After Argon, atoms again add a fourth shell, beginning with a 4s
orbital. Adding one electron makes Potassium (19) and two makes Calcium
(20). But then, before adding to the fourth shell,they add a new set of orbitals to the third shell. These are the 3d orbitals. There is one two-lobed 3d orbital (purple) and up to four four-lobed orbitals (orange). |
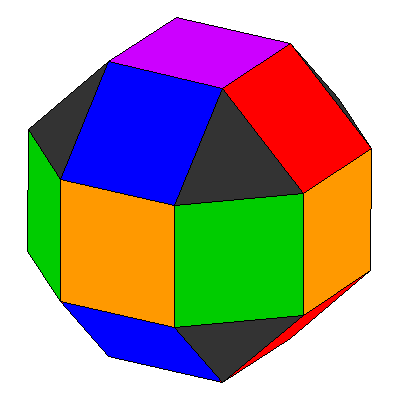 |
Trying to represent the d orbitals pictorially gets confusing. One way to picture the d orbitals Is like this. The square faces of a rhombicuboctahedron have the same symmetry as d orbitals. The two-lobed orbital extends through the purple faces at top and bottom, and the 4-lobed orbitals extend through the red, orange, green and blue sets of faces. |
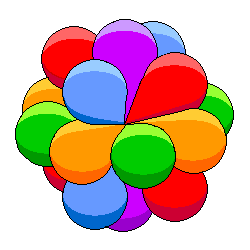 |
This is an attempt to represent the d orbitals of a transition metal. In all likelihood, the lobes all overlap just like the p orbitals above, but the resulting diagram would be all but impossible to understand. The coloring here and in the following diagrams is as in the diagram above. |
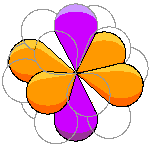 |
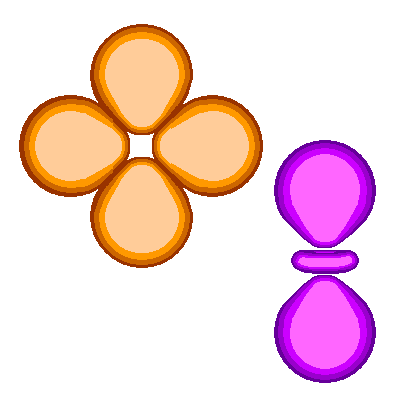
The orbitals fall into two groups. One of the four-lobed orbitals and the two-lobed orbital form one group. |
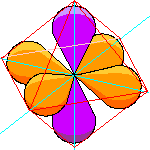 |
The lobes of these two orbitals have the symmetry of an octahedron. The actual nomenclature is a bit clumsy so we can call this the octahedral set. |
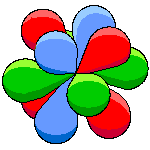 |
The other three orbitals |
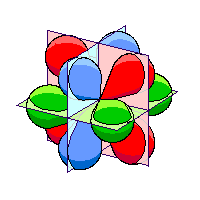 |
Each of the three orbitals lies in one of three perpendicular planes. Since the three orbitals are in orthogonal planes, we can call this the orthogonal set. |
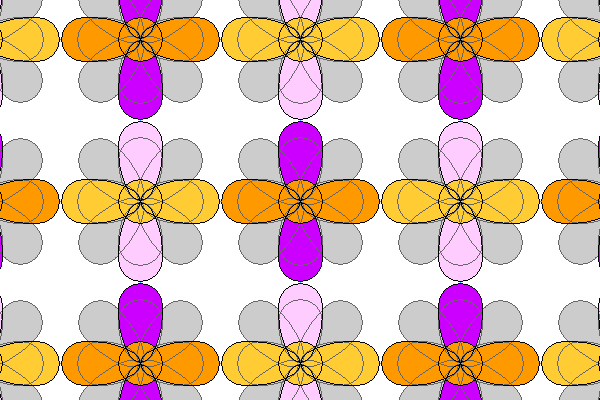
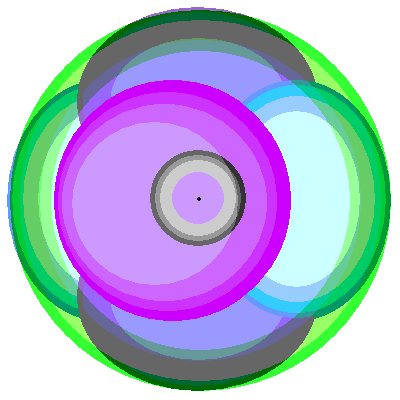
So here we have a possible simple atomic arrangement, with the cation (dark colors) and the ligand atoms or anions (light colors). What's holding the atoms together is the electrostatic attraction between the cations and anions. What's holding them apart is the Pauli Exclusion Principle that prevents electrons with identical quantum numbers from occupying the same space. (Also known as God's way of keeping everything from happening in the same place). Also the octahedral orbitals approach closely and have a strong electrostatic repulsion.
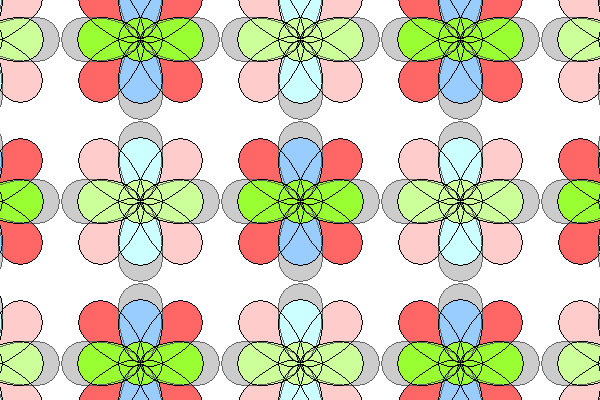
Above is the same atomic arrangement, this time showing the orthogonal p orbitals. The environment of these orbitals is quite different. The d orbitals are a lot further apart.
In addition to changing the energy levels of the orbitals, the crystal environment has another effect. Since the atoms in the crystal are vibrating due to thermal vibrations, the energy levels are smeared out into broad bands. An ion in a crystal that absorbed only in a narrow line would probably have little visible effect.
Transition Metal Compounds (Idiochromatic Colors)
Idiochromatic (self-colored) compounds owe their colors to an intrinsic ingredient of the compound. A classic example is the green and blue of copper compounds. Alythough copper is the best known, a few other transition elements are color-formers as well. Manganese produces reds and pinks, iron creates "earth-tone" colors as ferric iron (Fe+++) and greens as ferrous (Fe+2) iron. Nickel creates green colors.
As a cause of colors, idiochromatic transition metal colors are not very important. Much more common are allochromatic colors created by transition metal impurities.
 |
Now this is idiochromatic! An altar of malachite in St. Paul Outside the Walls in Rome. |
Transition Metal Impurities (Allochromatic Colors)
Allochromatic colors are not produced by an intrinsic ingredient of the material itself, but by impurities, either natural or artificial. All dyed materials are allochromatic. In minerals, pure quartz, corundum, beryl and feldspar are colorless, but they can assume almost any color when small amounts of impurities are present.
The principal simple test to tell allochromatic from idiochromatic colors is by the classic streak test. This is one of those seemingly absurd tests in mineralogy that actually is tied to a deep property. Idiochromatic colors are produced by an intrinsic ingredient of the material, so even fine particles are colored. Scraping the material against a fine rough surface, like an unglazed tile, rubs off a fine powder which still retains the color of the material. Allochromatic materials lack enough coloring to show up in fine powder, and give a white streak.
There are many other impurity-induced causes of color besides transition metal ions. Many transition metals that form complex anions (chromates, vanadates, and so on) owe their colors to charge transfer between the cations and oxygen. Ferric iron (Fe+++), probably the most important coloring agent in rocks, minerals, and soils, also owes its color to charge transfer. Colloidal particles and other inclusions create colors in milky quartz and feldspars. Dyes are mostly organic molecules.
Probably the most dramatic and celebrated case of allochromatic coloring is the red coloring of ruby and the green of emerald. Both are caused by chromium substitututing for aluminum, and both even have oxygen surrounding the chromium or aluminum in an octahedral configuration. Ruby is corundum (Al2O3) and emerald is beryl (Be3Al2Si6O18). The additional beryllium and silicon result in slightly weaker bonding in beryl, which has a hardness of 7.5-8 as opposed to 9 for corundum. This difference is not as slight as it might seem because, measured on a more precise scale, corundum is nearly twice as hard as beryl.
In ruby, there is strong absorption in the violet and the green-yellow part of the spectrum, some transmission in the blue and very strong transmission in the red. In emerald, as the energy levels of the chromium orbitals decrease, the yellow-green absorption drops into the red-yellow part of the spectrum, and the red transmission drops out of the visible range, and what's left is strong blue-green transmission.
Alexandrite is a gem variety of the mineral chrysoberyl (BeAl2O4). With, again, a small chromium impurity, it has transmission curves intermediate betwwn ruby and emerald. In strong blue light, the blue-green transmission predominates, but in red light (like incandescent or candle light) the red predominates.
Kurt Nassau and the Fifteen Causes of Color
Atoms and Light
Causes of Color: Incandescence and Simple
Excitations
Causes of Color: Energy Bands
Causes of Color: Molecular Orbitals and
Charge transfer
Causes of Color: Physical Optics
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update