Unusual Coordination
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
The most common coordination polyhedra, 4-fold (tetrahedral), 6-fold(octahedral), 8-fold (cubic) and 12-fold (close-packed) are highly symmetricaland tend to be associated with ionic or metallic bonding in which bonddirections are highly isotropic (uniform in all directions).
Other coordinations occur, and these tend to be associated with covalent bonding, which is often highly directional, or with irregular voids between clusters of atoms that have highly directional bonds. Many sulfide minerals have unusual coordination because of sulfur's covalent bonding. Many silicate minerals, especially nesosilicates, have unusual coordination created by voids between the silica tetrahedra (which have a mixture of ionic and covalent bonding.) Other minerals with tetrahedral radicals can also create unusual coordinations. The actual coordinations are rarely as symmetrical as the idealized polyhedra shown here.
4- and 5-fold
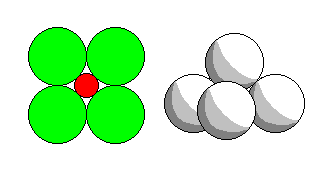
Square coordination and square pyramid coordination (found in the nickel sulfide millerite) has the same geometry as conventional octahedral coordination with one or two atoms removed.
Another way to achieve 5-fold coordination is with a trigonal dipyramid. Half of the aluminum atoms in andalusite are coordinated like this. Although technically any shape with 5 vertices and a triangle with points above and below is a dipyramid, in andalusite the dipyramids are somewhat irregular. The coordination is due to the andalusite being enclosed by octahedra and tetrahedra, and not to any inherent symmetry in the coordination bonding itself.
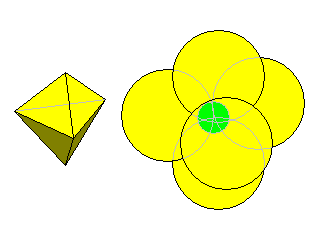
6-Fold
Molybdenite has 6-fold coordination in which the molybdenum atoms are surrounded by six sulfur atoms at the vertices of a triangular prism.
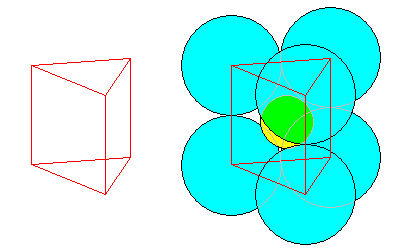
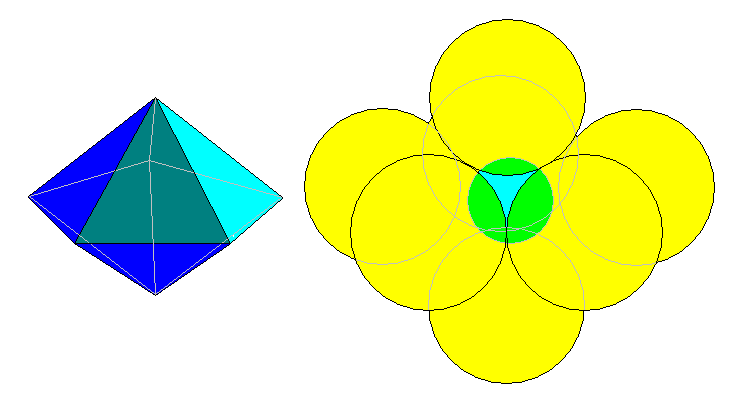
7-Fold
The most regular solid with seven vertices is a pentagonal dipyramid. If we stack spheres in that arrangement, we find that the central void is too flat to hold an atom. However, if we lengthen the dipyramid, it can serve as a coordination polyhedron. This is a geometrically possible packing but not a very likely one because there are no bonding schemes with the required five-fold symmetry.
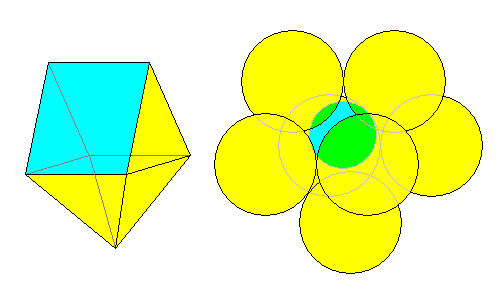
Another way to get 7-fold coordination is above. The coordination polyhedron is a square pyramid on one face of a triangular prism, sometimes called an augmented triangular prism. The atomic arrangement is at right.
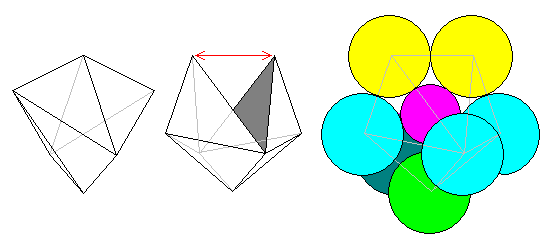
A slightly different approach is to start with a tetragonal disphenoid as at left and split it open as at center. The atomic arrangement is at right. This can also be considered a distorted pentagonal dipyramid. The calcium atoms in sphene have roughly this coordination.
8-Fold
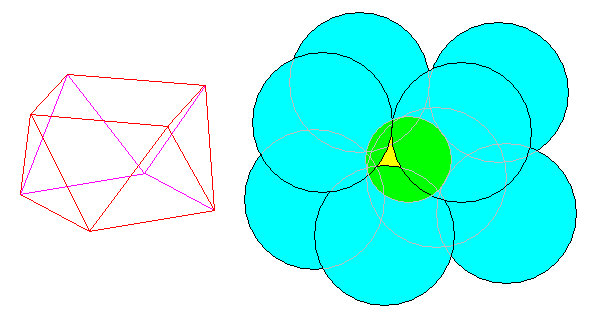
Scheelite, the calcium tungstate, has its tungsten atoms in tetrahedral coordination with oxygen. The calcium atoms are enclosed by eight oxygen atoms at the corners of a square antiprism (actually a bit distorted - see below). Opposing faces are squares rotated by 45 degrees with respect to each other and their vertices are connected by equilateral triangles.
Another very important 8-fold irregular coordination polyhedron is based on a solid called the snub disphenoid. (Snub is a geometrical term referring to faces that are regular polygons, usually triangles, but not related to asymmetry axis.) A snub disphenoid can be created by separating opposing pairs of tetrahedron faces and joining their vertices with a zigzag belt of equilateral triangles. The resulting solid has 4-fold inversion symmetry (actually 4*m2).Since the original tetrahedral face angles are distorted, we call the shape a snub disphenoid rather than a snub tetrahedron.
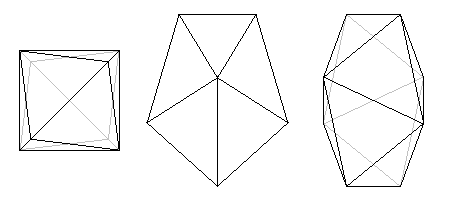
Above, left, is a view down the symmetry axis. At right are two side views. The center view is along a mirror plane and the right view is along a two-fold symmetry axis. The solid has 12 equilateral triangular faces.
The center view looks a bit like a distorted pentagonal pyramid. Another way to picture this solid is to begin with a pentagonal dipyramid, cut two adjacent equatorial edges, squeeze from the sides to open the cut, and insert two equilateral triangle faces in the gap.
This solid is too elongated to be a coordination polyhedron, but distorted forms that are more equidimensional are common. A typical shape is shown below.
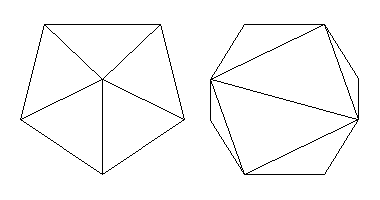
Many of the complex 8-fold coordinations, like those in garnet, that are described as "distorted cubes" are actually this shape. After all, if you bisect the six faces of a cube diagonally, you get a form with 12 triangular faces. This is actually the most general 8-fold coordination polyhedron. The "square antiprism" coordination polyhedron of scheelite, above, is more exactly a very flattened version of this polyhedron.
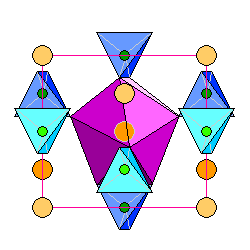 |
At left is a view of the zircon structure, showing the central zirconium atom enclosed in a distorted snub disphenoid (purple). |
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 9 May 2002, Last Update