Cryolite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Cryolite Na3AlF6 is a rare mineral, mostly of interest because of its role in aluminum smelting.
For a mineral with a simple formula it has a somewhat irregular structure. Because of the high charge of aluminum and the small center to center distance between aluminum and fluorine, the aluminum atoms are octahedrally coordinated with the fluorine and the octahedra are quite regular. The sodium ions can't compete in either size or charge and they occupy voids between the aluminum octahedra, in two sets. One set has fairly regular octahedra larger than the aluminum octahedra, and the other occupies the voids between the octahedra and has irregular coordination polyhedra.
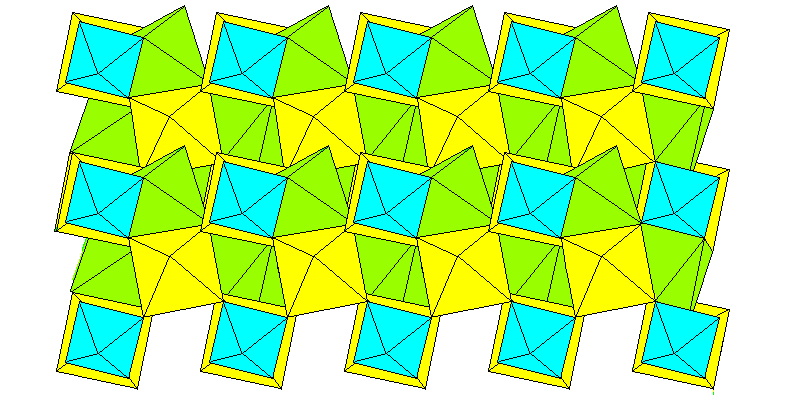
Above: view down the c (pseudo-tetragonal) axis. Aluminum octahedra are blue, sodium octahedra are yellow, irregular sodium polyhedra are green.
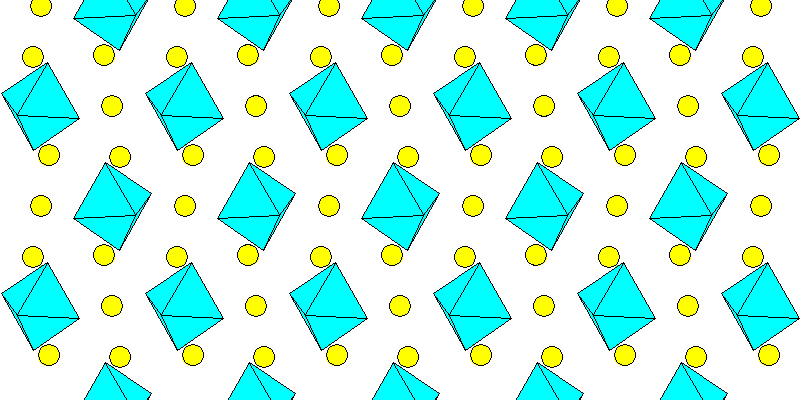
Above: Aluminum octahedra and sodium atoms (yellow)
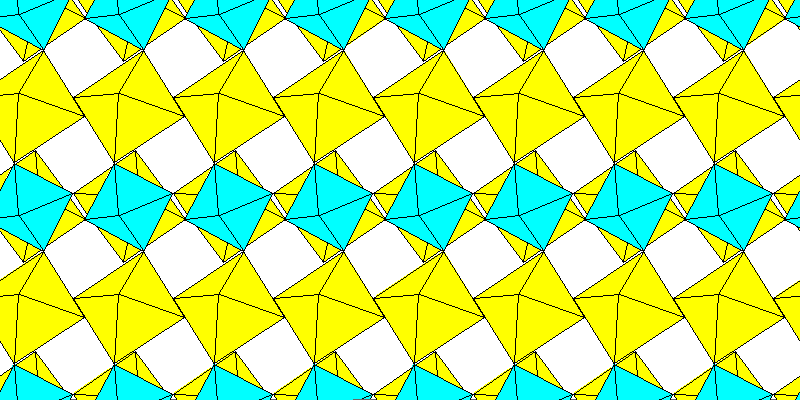
Above: Aluminum and sodium octahedra.
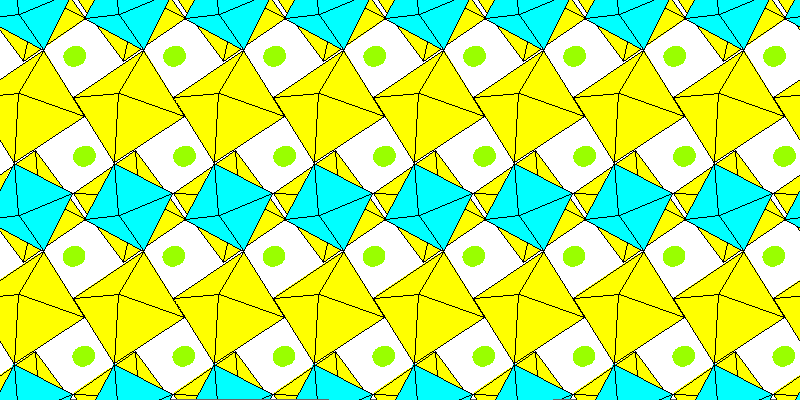
Above: Aluminum and sodium octahedra. Remaining sodium atoms in green.
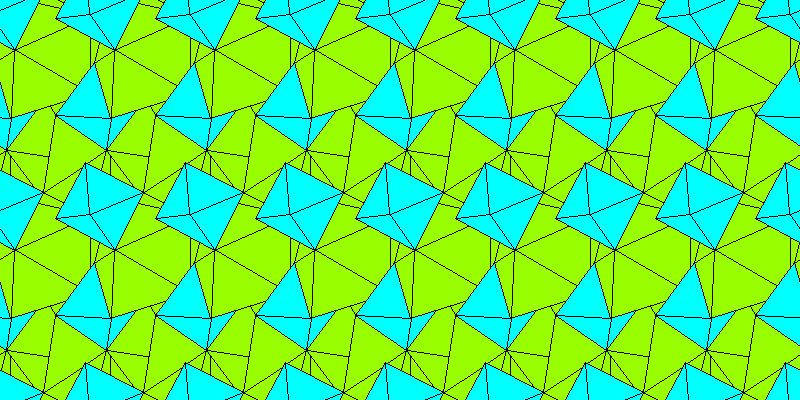
Above: Aluminum octahedra and irregular sodium polyhedra.
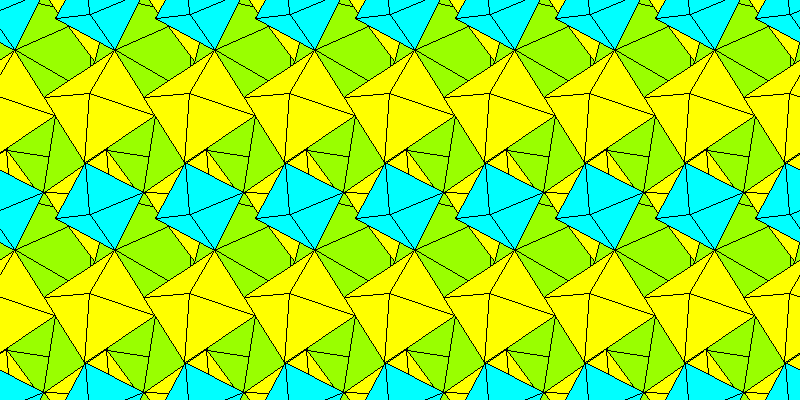
Above: Complete structure.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update