Diamond Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
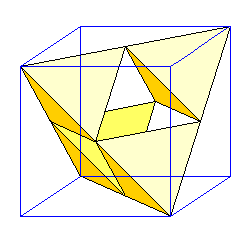 | Numerous mineral structures are based on the fact that tetrahedra can be inscribed in a cube. If atoms have a face-centered arrangement, we can join a corner atom to the three nearest face-centered atoms to create a tetrahedron. Four similarly-oriented tetrahedra can be created in the cube. |
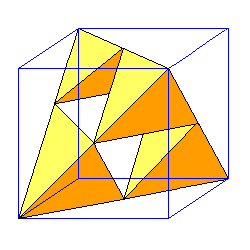 | There are two ways to orient tetrahedra in a face-centered cubic array. |
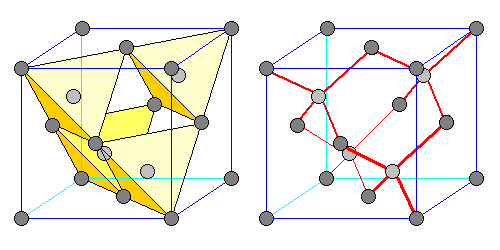 |
| Diamond is one mineral that employs this structure. There are carbon atoms in a face-centered array (dark gray) plus an extra one (light gray) at the center of each tetrahedron. At left the relationship of the carbon atoms to the tetrahedra is shown. On the right the carbon-carbon bonds are shown. |
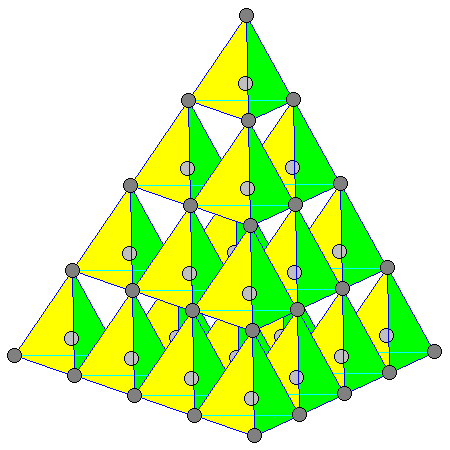 | At left is the diamond structure in a different orientation showing the tetrahedral structure a bit more clearly. As above, carbon atoms at the corners of the tetrahedra are dark gray, those in the middle are light gray. |
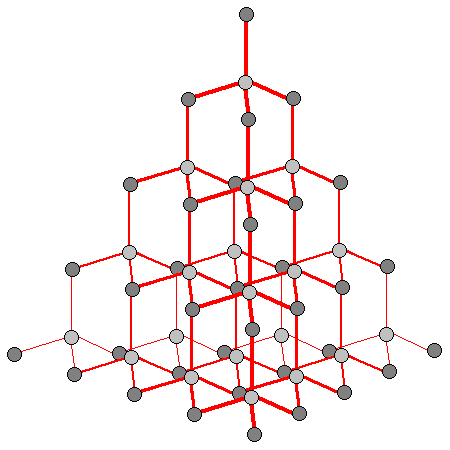 | At left is the diamond structure showing the carbon-carbon bonding. Bonds closer to the viewer are shown thicker. |
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 February, 2001, Last Update