Feldspar Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
For such an essential group of minerals, the textbook descriptions of feldspar are frankly a complete mess. No two texts present the structure the same way, and none really provide enough alternative views to give a clear picture of the structure.
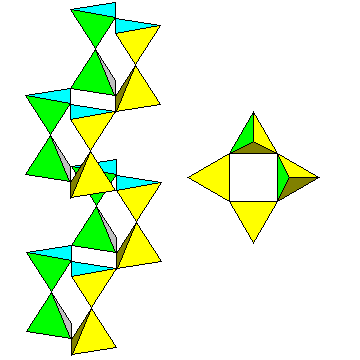 |
Most textbooks start off by describing the zigzag paired chains that make up the structure. The chain, which runs parallel to the a axis, is shown at left.
A view along the chain is at right. |
Below is a view of feldspar in the (010) plane, along the b-axis, with the a-and c-axis directions shown. For all the feldspars, the a-c angle is close to116 degrees. The structure can also be considered to consist of double layers of four-membered rings. The upper layer is yellow, the lower blue. Each double layer is bounded above and below by a horizontal mirror plane.
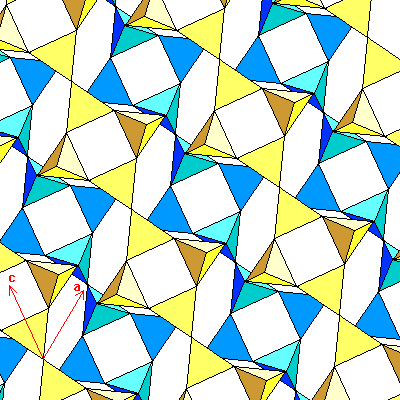
The same view is shown below, colored to show the double chains. Light shades denote the upper layer, dark shades the lower. Unlike the first diagram of the chains, the chains here are horizontal and seen face-on, or lying on their backs. The feldspars have cleavage along (001), that is, the vertical plane between the chains, and (010), the mirror planes between the double layers.
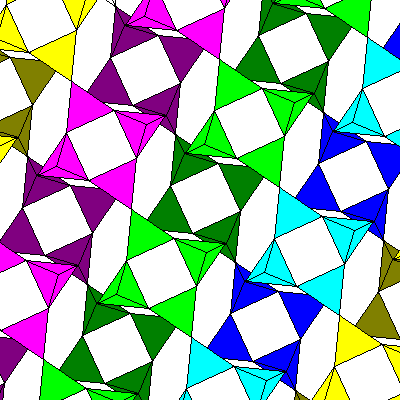
Below is a view looking down the a-axis, showing cross-linkages between the chains. The chains enclose elongated voids which contain pairs of large cations. The oxygen atoms midway along the elongate voids pinch the void off into a "waist" that permits it to cage two cations.
The chains are colored to help identify them. The green and blue chains are mirror images of the yellow and orange ones. Cations are in purple, with darker shades farther below the plane of the diagram. The chains are not perfectlystraight but have slight twists. Each set of tetrahedra in the chain is related to the one above and below by a vertical glide plane with translation perpendicular to the plane of the diagram. Mirror planes and vertical glide planes alternate, with horizontal two-fold rotation axes perpendicular to both.
We're looking along the [100] direction here, but since the a-c angle is 116degrees, the plane of the diagram is not (100) but (201).
Varieties of Feldspar
The general formula for the feldspars is XAl(Al,Si)Si2O8,where X is potassium, sodium, calcium, or barium. The Al occupies some of the tetrahedra. All the X ions are big, much larger than iron or magnesium, which are too small to occupy the voids in the structure fully. The radii of the principal feldspar cations in Angstrom units are: K 1.6; Ba 1.5; Na 1.4 and Ca1.25. Also, Ba and Ca are divalent, but Na and K have a valence of +1.
The factors that influence feldspar structure are:
- Ionic radius
- Charge balance
- Ordering of aluminum-silicon tetrahedra, and also ordering of the large X ions. The ordering of Al and Si tetrahedra depend on temperature and the number of Al necessary to maintain charge balance. If there are both +1 and +2 X ions, the +2 ions will tend to avoid areas of high Si (+4) concentration
From the above we can expect K, Ba and Na to be most compatible based on ionic radius, and the K-Na and Ba-Ca pairs to be compatible based on charge. We might expect the most compatibility between K and Na and the least between K and Ca. Since Na is in the middle of the ionic radius spectrum we might expect it to be compatible with all the other ions. It turns out, from actual mineralogy, that ionic radius is most important and charge balance and ordering are secondary.
The barium feldspars are quite minor and consist of celsian (BaAl2Si2O8) and hyalophane ((K,Na,Ba)(Al,Si)4O8), a general solid solution of potassium, sodium and barium feldspar. This is exactly the relationship we would expect based on ionic radius.
The potassium feldspars (KAlSi3O8) differ in the ordering of Al-Si tetrahedra. At high temperatures the tetrahedra are randomly mixed (although Al-O-Al links never occur) and the lattices are therefore of high symmetry. They have long range order even if on a local scale the lattice is disordered. Sanidine is the highest temperature variety and is completely disordered, orthoclase forms below about 800 C and is partially ordered, and microcline forms below about 600 C and is highly ordered. The complete ordering of Al in microcline breaks down the mirror plane symmetry and microcline becomes triclinic. The a-b and b-c angles of the unit cell depart from 90 degrees by a fraction of a degree (that's what micro-cline means - small angle).
Potassium feldspar (KAlSi3O8) and the sodium feldspar albite(NaAlSi3O8) are completely compatible at high temperatures. Sanidine can contain up to 60% Na, a mixture of K and Na-feldspar that is 60-90% Na is termed anorthoclase. However, at low temperatures the difference in ionic radius begins to tell and the feldspar separates into discrete streaks and patches of K and Na feldspar. This process is called exsolution and is analogous to cream separating from milk. If the dominant feldspar is K-feldspar the resulting mixture is termed perthite, and if it is albite the mixture is termed antiperthite.
Despite the difference in charge, Na and Ca are not that different in ionic radius, and there is a complete solid solution series, the plagioclase series, between albite and anorthite (CaAl2Si2O8).The different members of the series are arbitrarily divided at 10, 30, 50, 70 and 90 per cent anorthite. The different members are:
- Albite
- 0-10% anorthite. Found only in very sodic rocks, hence usually metamorphic or formed in marine conditions as a sedimentary cement, or by ion exchange with more calcic plagioclase.
- Oligoclase
- 10-30% anorthite. The dominant plagioclase in granitic rocks
- Andesine
- 30-50% anorthite. Found in intermediate igneous rocks
- Labradorite
- 50-70% anorthite. The dominant plagioclase in gabbro and basalt. Also, despite their name, most anorthosites are made up of labradorite.
- Bytownite
- 70-90% anorthite. The rarest. Requires both a lot of calcium and also significant sodium. Most igneous settings have too much sodium, most calc-silicate metamorphic settings have too little sodium.
- Anorthite
- 90-100% anorthite. Generally a metamorphic mineral in calc-silicate rocks.
The plagioclases are triclinic and their a-b and b-c angles are a bit more oblique than microcline. Hence the name: plagio-, oblique and clase, break.
Since Na and Ca differ in valence, Al has to substitute for Si to compensate. The Al-Si orderings of albite and anorthite are different, and at low temperatures, plagioclases in the middle of the composition range also exsolve, but on a submicroscopic scale. These submicroscopic textures are probably responsible for the iridescence of some plagioclases.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 13 March, 2002, Last Update