Hydrohalite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Hydrohalite (NaCl•2H2O) is one of a group of minerals we might dub "musherals" because they are 50% or more water. It is described in many mineralogy books and sites as "very rare." It really isn't, in the sense of many other minerals that require extremely restricted physical or chemical conditions to form. It's significant for three reasons. First, whenever a mixture of NaCl and brine freezes, much of the brine freezes to make hydrohalite. Thus, it's rare in the sense we don't often see concentrated brine freeze, but where it does happen, like Antarctica, hydrohalite is common. Second, hydrohalite doesn't melt. It breaks down at 0 C to brine and NaCl (halite), or "sweats" out the water. Thus it's possibly the easiest example of incongruent melting to understand. Third, while conditions for the formation of hydrohalite might be uncommon on Earth, they are probably very common on Mars and the interiors of icy satellites.
The structure shown here is idealized. In actual hydrohalite the rings of sodium atoms and the enclosing octahedra are somewhat irregular.
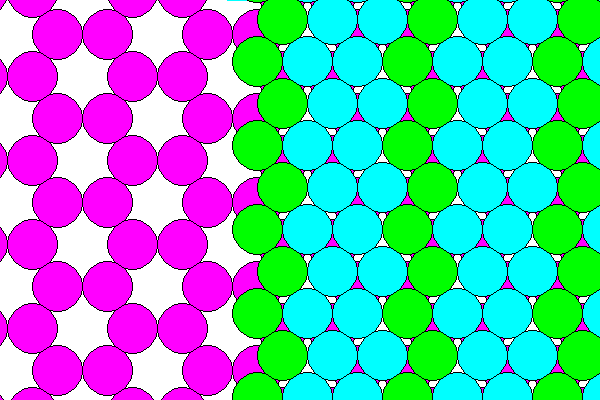
Hydrohalite is not halite with interstitial water, nor is it ice with interstitial Na and Cl. It has a structure distinct from both. It consists of sheets of Na, Cl and water. The middle of each sheet consists of a hexagonal network of Na ions (magenta). The Na layer is held together by zigzag chains of Cl ions (green) and water molecules, with the negative oxygen ions (blue) pointing toward the sodium sheet. Note that these illustrations are schematic to show the fundamental geometry. In reality the sodium sheet is not truly hexagonal nor flat. The sodiums are octahedrally coordinated with two adjacent neighbors being chlorine and the rest water. In real hydrohalite the octahedra are also not completely regular. It differs from many other "musherals" in not having cations completely enclosed by water.
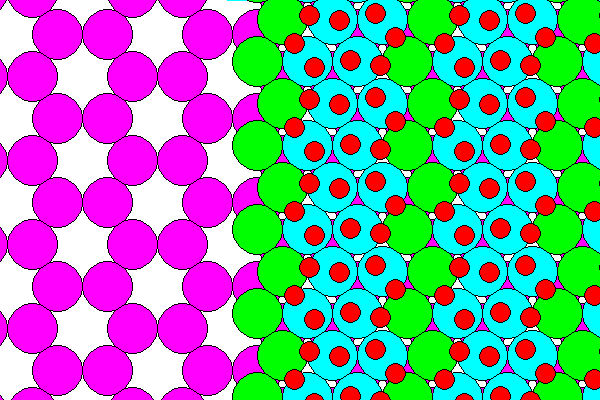
The hydrogen ions (positive, red) point away from the sodium sheet. Each water molecule has one hydrogen bonded to a chlorine atom and the other pointing outward, as shown above
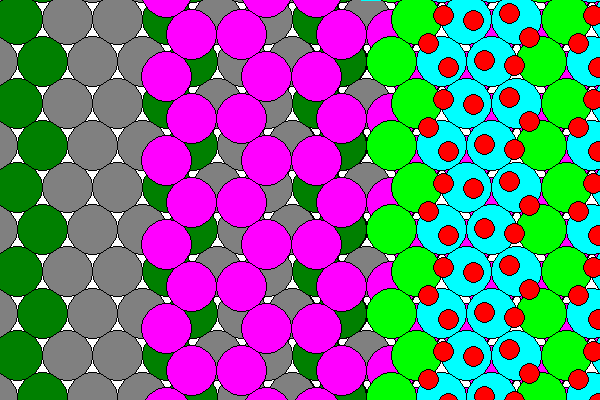
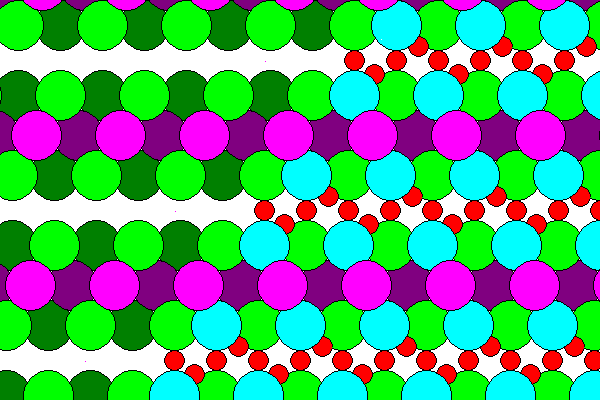
The diagram above shows the lower layer of Cl and water in dark colors, the sodium layer in magenta and the upper layer in green, blue and red. None of the lower hydrogens are visible because they are on the opposite side of the sheet. The sodium sheet has approximate six-fold symmetry but the arrangement of chlorine and water does not. Also adjacent layers are offset relative to each other, so hydrohalite only has monoclinic symmetry. The two-fold rotation axis runs horizontally across each figure above.
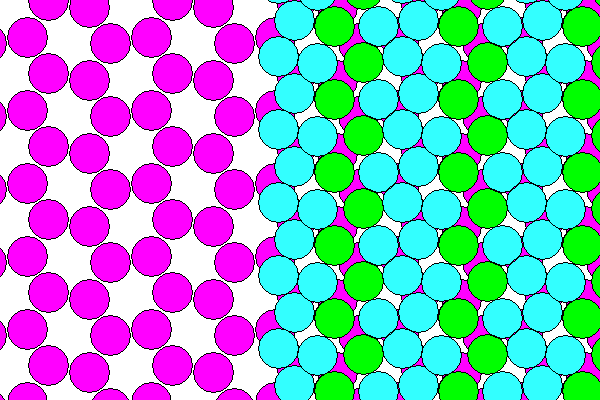
The diagram below shows the actual arrangement of the atoms. The sodium hexagons are slightly distorted as are the water-chlorine layers.
The diagram below shows an idealized view of the coordination polyhedra. Sodium atoms in purple are at the centers of the octahedra. Chlorine atoms are in green, with those on the bottom of the layer in lighter color, and partially hidden. Unlabeled vertices are occupied by water molecules. We can see this is basically a dioctahedral sheet.
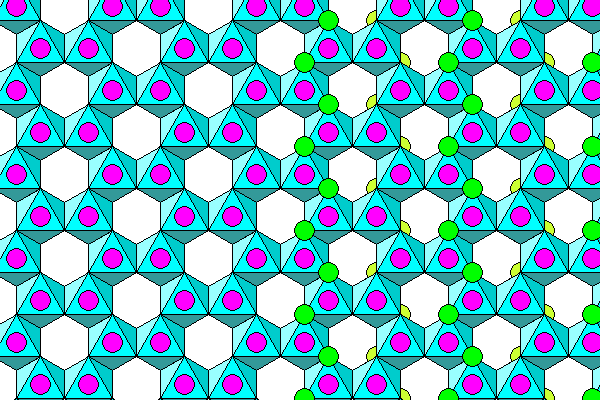
Below is a more realistic depiction. Atoms are reduced in size. Sodium is purple, chlorine is green, the oxygens of water molecules are blue and the octahedra are yellow.
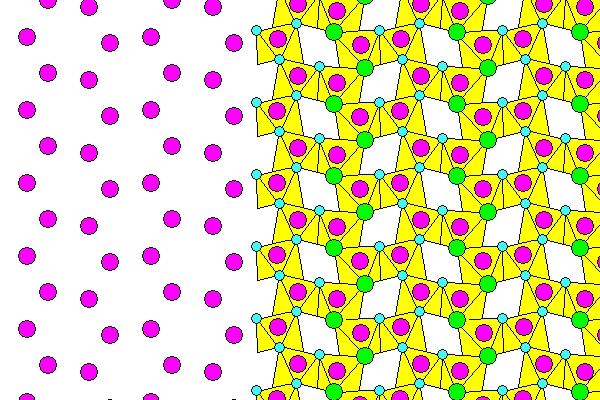
Looking along the twofold symmetry axis, above. The left side of the diagram shows sodium and chlorine only; the right side shows water molecules in front of the rear chlorine atoms. Foreground atoms are in light colors. The outward pointing hydrogen ions link the sheets together. The other hydrogen in each water molecule connects the oxygen and chlorine atoms. There does not appear to be any published information of the cleavage of hydrohalite, but we can expect it to be very good in the [100] plane between the layers.
Hydrohalite is one of a group of minerals we might dub "musherals" because they are 50% or more water. Another hydrous mineral, CaCO3.H2O, was formerly called "hydrocalcite," but is now called "monohydrocalcite." It seems likely that hydrohalite will soon be called "dihydrohalite," as well.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 Sept 1997, Last Update