Malladrite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences,
Malladrite Na2SiF6 is one of the few minerals that contains silicon but is not a silicate. Most of the silicon-bearing minerals without oxygen are either elemental alloys, carbides, nitrides or halides.
For a mineral with a simple formula it has a somewhat irregular structure. Because of the high charge of silicon and the small center to center distance between silicon and fluorine, the silicon atoms are octahedrally coordinated with the fluorine and the octahedra are quite regular. Also, fluorine is smaller than oxygen, favoring octahedral coordination. The sodium ions can't compete in either size or charge and they occupy voids between the silicon octahedra, with irregular octahedra.
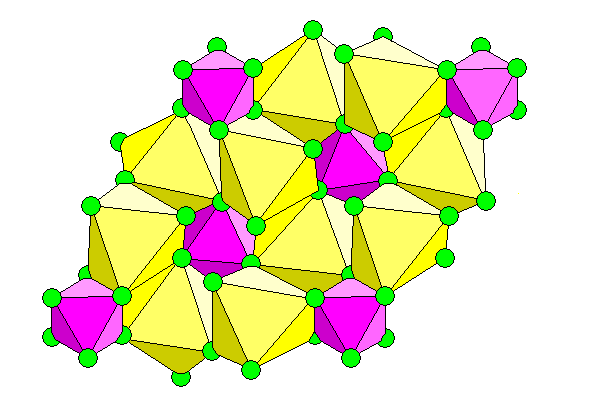
Above: View down the c-axis. Silicon polyhedra are purple, sodium yellow, and fluorine atoms (smaller than scale) are green.
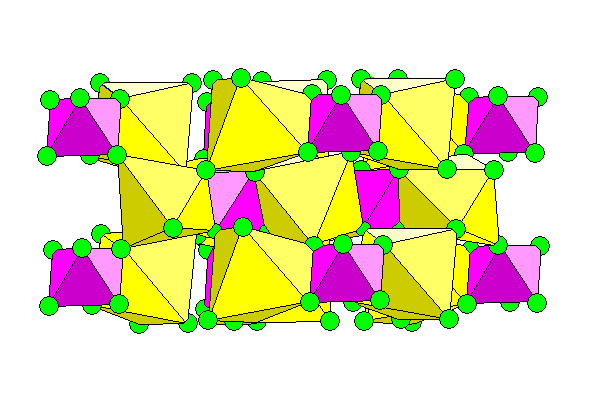
Above: view perpendicular to the c-axis.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update