Pectolite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
In terms of its chemistry and associations, pectolite seems akin to the zeolites, but it is actually a pyroxenoid. It's a silicate of calcium and sodium, both large cations, and therefore has to deal with the size issue much the same way that wollastonite does, by canting the tetrahedra until they link up. But unlike wollastonite, pectolite includes sodium.
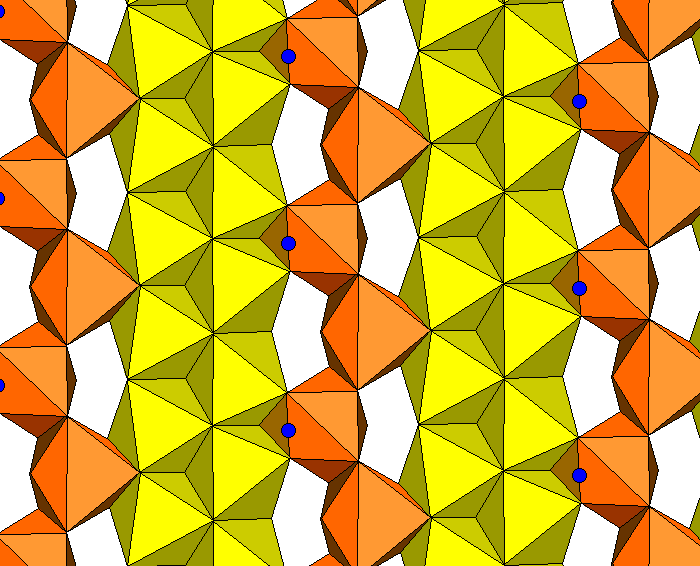
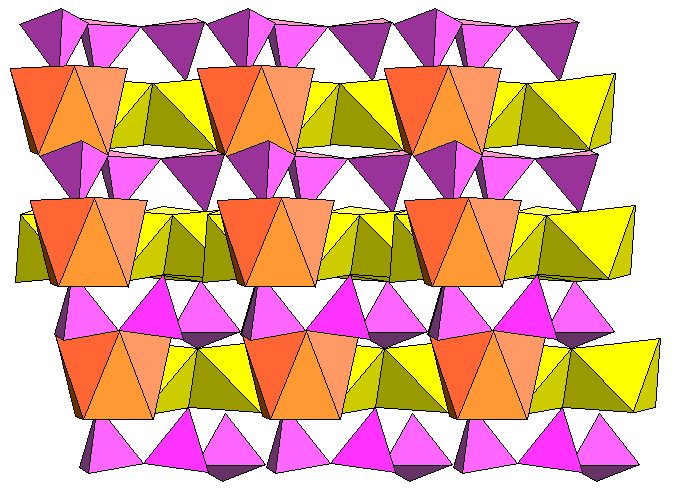
The sodium atoms separate strips of Ca-O octahedra. In the diagram below, yellow polyhedra are filled with calcium, orange with sodium. Blue spheres are hydrogen atoms because some oxygen locations are occupied by hydroxyl.
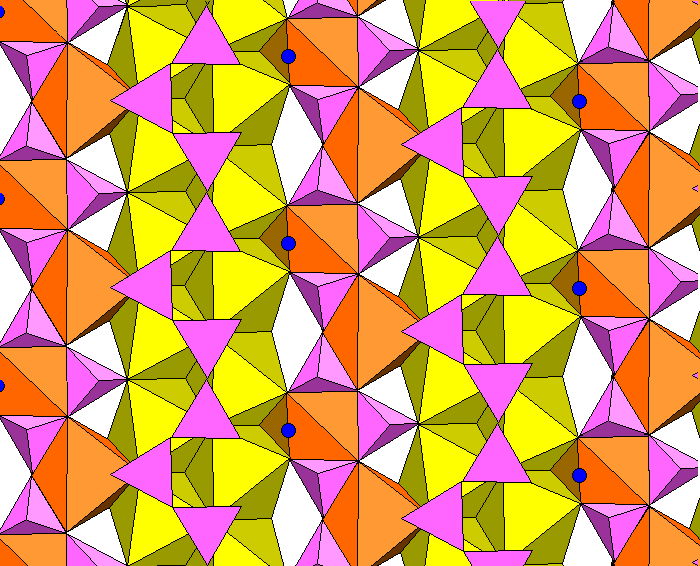
The figure below shows how the silica chains (pink) are kinked to accommodate the large cation polyhedra.
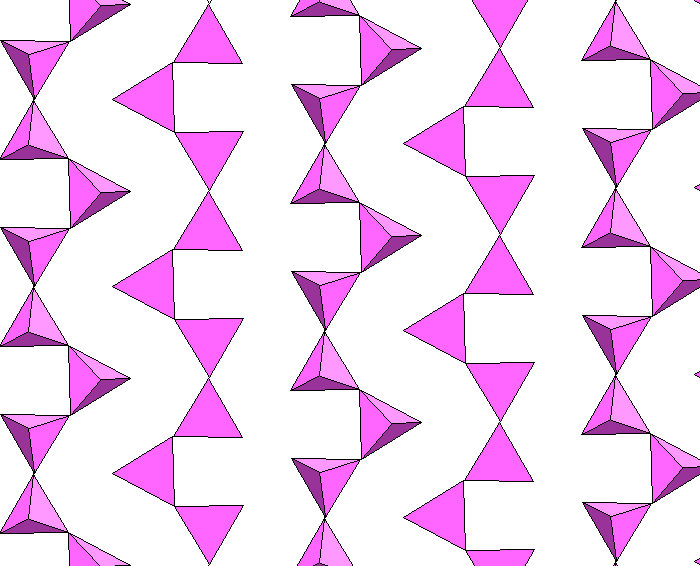
Below, the silica chains are shown in isolation.
Below, an end-on view of the structure.
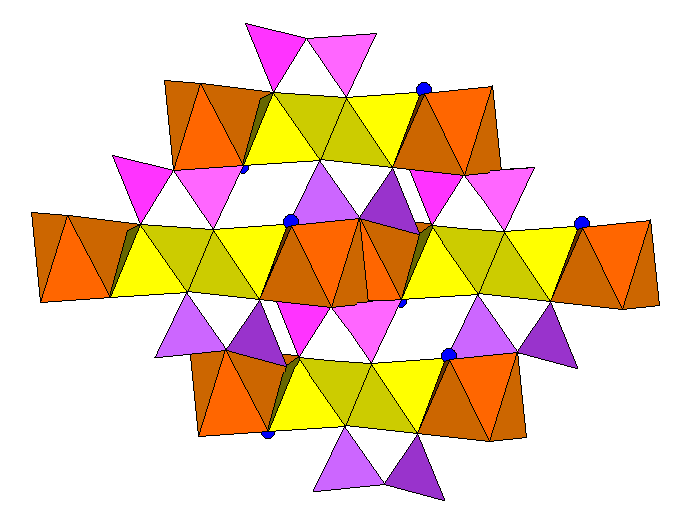
Below, longitudinal view of the structure, showing the canting of the tetrahedra.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 15 October 2009, Last Update