Perovskite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Perovskite is a relatively minor mineral with the formula CaTiO3. From its formula, we might expect it to form in environments rich in Ca (like carbonate rocks) and Ti (mafic igneous rocks) but poor in Fe (otherwise the titanium would go into ilmenite) and poor in Si (or the titanium would go into sphene). Typical environments include contact metamorphic rocks where carbonates are intruded by mafic rocks, nepheline syenites, and carbonatites.
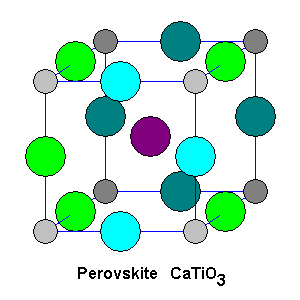 |
The simplest way to picture perovskite is a cubic unit cell with titanium atoms at the corners (gray), oxygen atoms at the midpoints of the edges (green and blue), and a calcium atom (purple) in the center. Dark shades are used to indicate layers further back. |
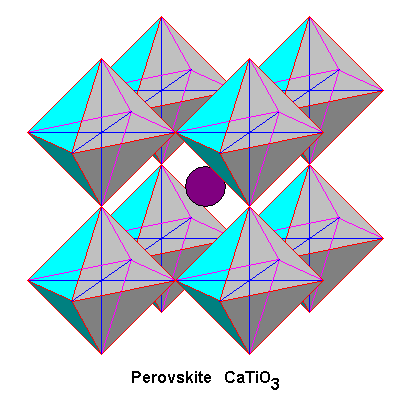 |
Each titanium atom is octahedrally coordinated with six oxygen atoms, with a calcium atom in the center. Although the structure is ideally cubic, the actual mineral is very slightly monoclinic because one of the unit cell angles is 90 degrees 40 minutes. |
Perovskite isn't a terribly important mineral in its own right, but recall that magnesium pyroxene (enstatite) has the formula MgSiO3. Thus we can rearrange pyroxene to be isostructural with perovskite, a theoretical concept that has recently been experimentally verified. In this structure, Mg would assume the role of Ca, and Si would be in the Ti positions (note that it would not be tetrahedrally coordinated). This probably happens at a depth of several hundred kilometers in the mantle. Since much of the mantle is composed of ferromagnesian silicates, this polymorph of MgSiO3 could be the single most abundant mineral in the Earth.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 Sept 1997, Last Update