Rosenbergite
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Rosenbergite, AlF3�€ï¿½3(H2O), earns an "honorable mention" as a musheral because it doesn't quite have as many water molecules as other atoms. It has four atoms in its formula versus three water molecules. Nonetheless it's pretty watery. In fact, the illustration at webmineral.com is too watery because there aren't enough fluorine atoms shown to balance the aluminum.
The reason becomes apparent in the original reference, which lists the formula as AlF[F0.5(H2O)0.5}4.H2O. The reason for the weird fractional formula units, which could be easily converted to whole numbers, is to indicate there are four sites where there is a 50-50 chance of having either water or fluorine. Since two opposite atoms in each coordination octahedron are fluorine, the four remaining sites are the equatorial atoms around the middle. In the diagrams shown here, these are shown in an ordered arrangement, but the arrangement probably need not be. It has a lovely symmetrical tetragonal structure with chains of octahedra linked by free water molecules.
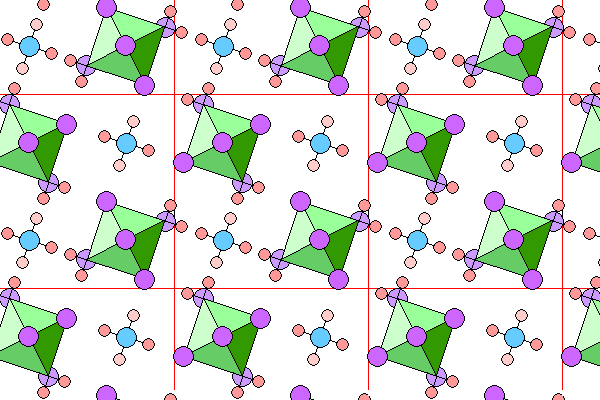
Below is a view down the c-axis. Aluminum octahedra are in green, fluorine atoms in violet with foreground atoms darker. Hydrogen atoms are pink, with foreground atoms darker. Oxygen atoms are not shown for the corner water molecules in the octahedra, but are in blue for the interstitial water molecules. Because of the high symmetry, foreground and background atoms overlap. The light fluorine atoms are on the next octahedron down, and the light hydrogen atoms are on the next water molecule down.
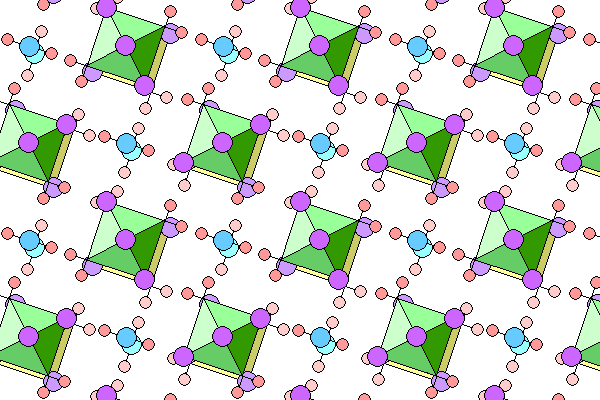
Below is a slightly oblique view to show the lower level of octahedra (yellow) and water molecules. All other colors are as in the diagram above.
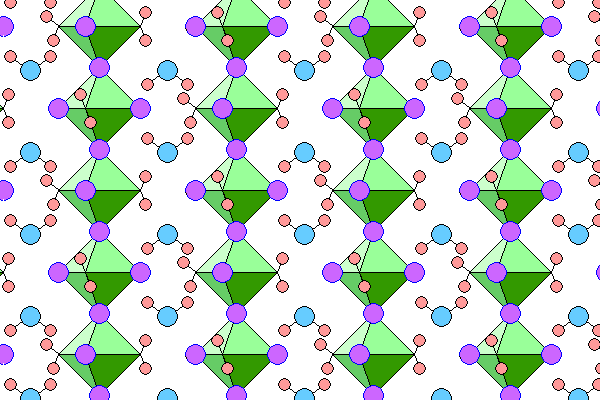
Below is a side view of the structure with the c-axis vertical. The view is along the closest spaced planes of octahedra so is oblique to both the a and b axes.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 28 March 2011, Last Update