Sillimanite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Sillimanite is a nesosilicate and the high temperature form of Al2SiO5. Even though it contains chains of tetrahedra, the chains are alternately filled with silicon and aluminum atoms. For a relatively simple structure it can be hard to visualize and depict.
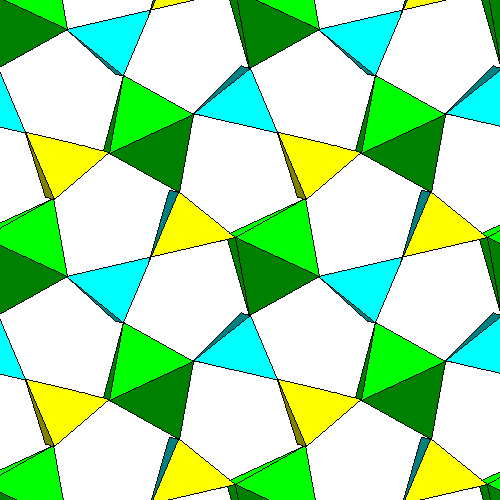 |
Sillimanite consists of chains of aluminum octahedra (green) joined by alternating silica and aluminum tetrahedra (yellow and blue).
The a and b dimensions of the sillimanite unit cell are nearly the same and the pattern of octahedra has nearly fourfold symmetry. However, the intervening tetrahedra do not have fourfold symmetry and the overall structure is orthorhombic. Each group of four chains encloses a flattened octagon, and pairs of tetrahedra bridge the short diagonal. The tetrahedra bridge the gaps between points of neighboring octahedra. In this view the vertical edges are inclined slightly to make all the tetrahedron vertices visible. |
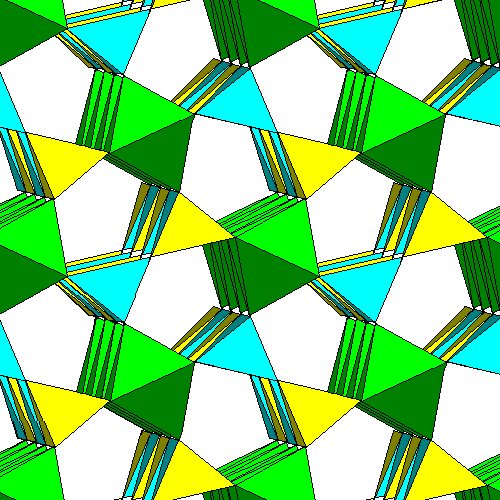 |
This view, slightly oblique to the c axis, shows four layers of the structure. Note how the tetrahedra bridge the gaps between the octahedra and alternate. |
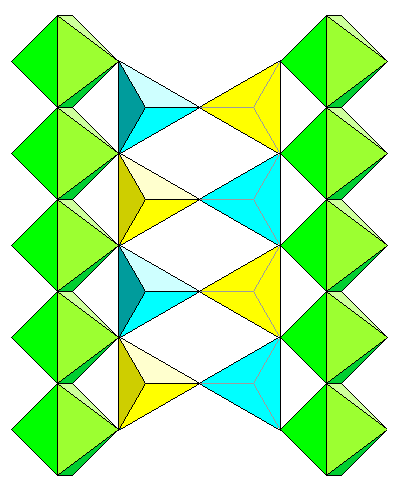 |
The view at left shows the chains of octahedra from the side, with tetrahedra bridging the gap between points. The view is along the a axis. |
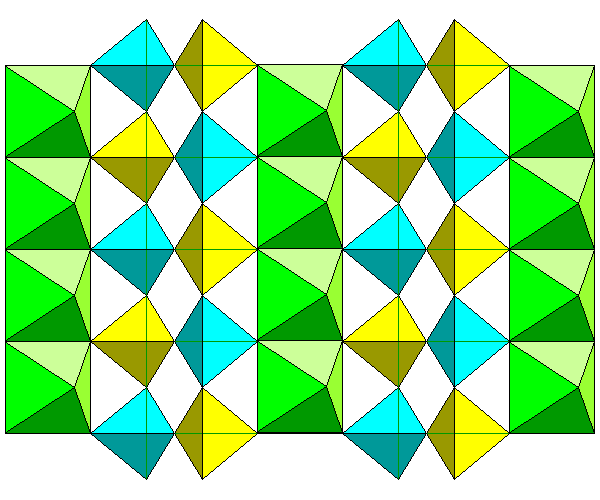
The view above shows the chains of octahedra from the side. The view is along the b axis. Here we see single tetrahedron vertices linked to two tetrahedra. The vertical edges of the tetrahedra join to other chains of octahedra above and below the plane of this diagram.
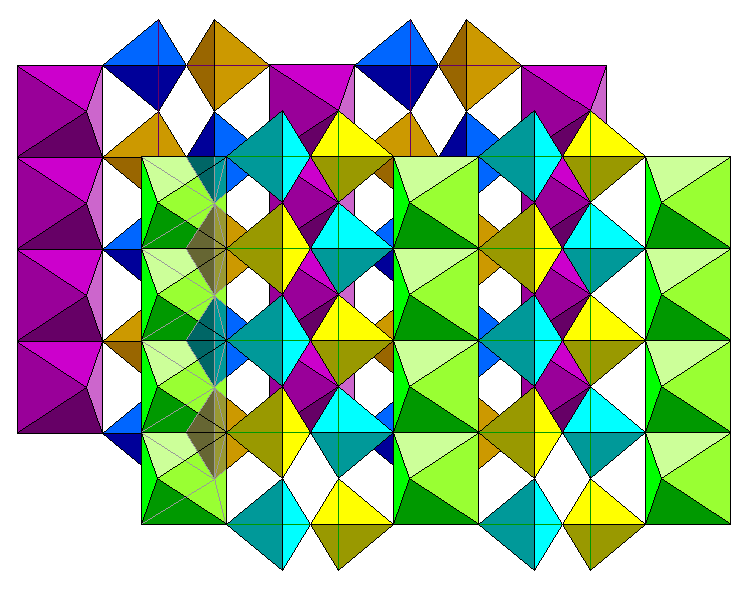
Here we see two layers of the structure with the rear layer in darker colors. The leftmost chain of octahedra in the front layer is rendered transparently to show how the rear tetrahedra connect to the front octahedra.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 5 April 2005, Last Update