Solubility Constants
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Gypsum, Cops and Robbers
A good simple geological example of mineral solution is calcium sulfate. Technically, pure calcium sulfate is anhydrite and the more familiar gypsum is hydrated calcium sulfate. Since we're talking about what happens in water, we can safely call the resulting product gypsum. We can picture what happens with the following analogy.
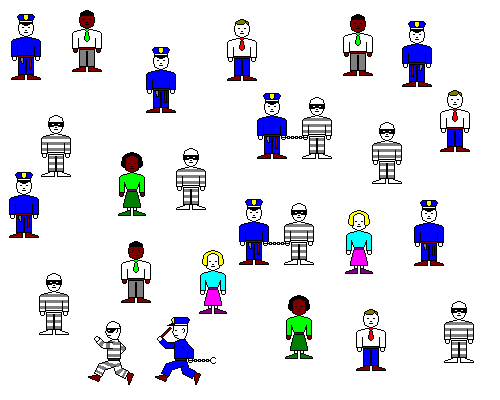
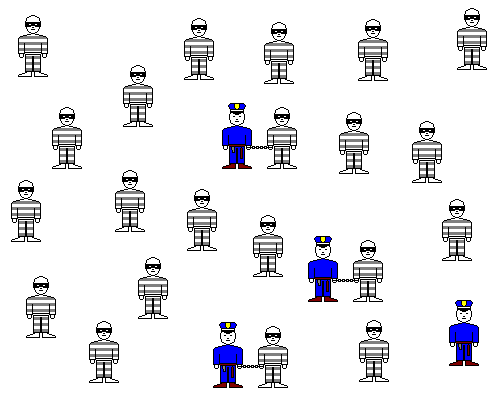
Let's imagine the Ca ions are cops and the SO4 ions are robbers. When a cop and robber meet, they get handcuffed together. However, occasionally a robber breaks free of the cuffs. The Ca+2 and SO4-2 combine to make [relatively] insoluble calcium sulfate, but some of the Ca and SO4 ions return to the solution. This little thought experiment reveals several points.
- It's how sparsely the cops and robbers are spread out, not their absolute number, that's important. A given number of cops and robbers spread out over a whole county won't result in the same number of arrests as if they were all confined to the same small town. Similarly, it makes a difference whether the Ca and SO4 ions are dissolved in a thimble or in a swimming pool.
So it's the number of atoms or molecules per unit volume that's important, not weight per se. The measure of concentration is called molarity and is the number of moles per liter of solution. Since atoms and molecules have differing weights, we use a unit that contains a fixed number of atoms or molecules: a mole. It's a gram-formula weight - the molecular weight of the molecule in grams. For example, calcium has an atomic weight of 40, so a mole of calcium is 40 grams. Water has a molecular weight of 18 (16 for the oxygen, one each for the hydrogens) so a mole of water is 18 grams. A mole of anything contains 6.02 x 1023 atoms or molecules. - The presence of law-abiding citizens has little effect. Similarly, if we dump potassium or nitrate ion into the solution, neither of them will react with the calcium or the sulfate, and their presence doesn't count (to first approximation, but see below).
- However, just like a dense crowd can impede policemen trying to pursue a criminal, concentrated solutions can be a bit more complicated. If there's so much dissolved material that it affects the overall volume, then you have to take it into account. For example, if you mix half a liter of sulfuric acid and half a liter of water, you can't proceed as if you had sulfuric acid dissolved in a liter of water. (The amount of water that goes into the gypsum isn't enough to affect the chemistry significantly.)
- Also - think of the movie Dog Day Afternoon - if a crook is completely surrounded by innocent citizens, the cops can't see him. Organic acids are good at surrounding ions and effectively isolating them from the solution. Another ion is then free to enter the solution in its place. This is one way organic acids help weather rocks.
- Finally, a crook might hide in plain sight by striking up a conversation or tagging along with a law-abiding citizen. And a cop who's distracted by chatting with people on the street won't catch many crooks. Similarly, if you dump a lot of sodium chloride into water with calcium and sulfate already in it, some of the sulfate ions will be attracted to sodium, and some of the calcium ions to chlorine, and neither will be readily available for forming gypsum. The actual effect of ions in solution is not always what their concentration would suggest and is called their activity.
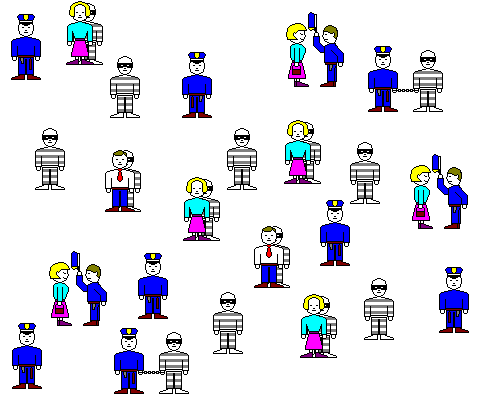
In the sketch above, some of the cops are distracted and robbers are hiding behind civilians. The interactions of the cops and robbers won't be the same as if there were only cops and robbers around.
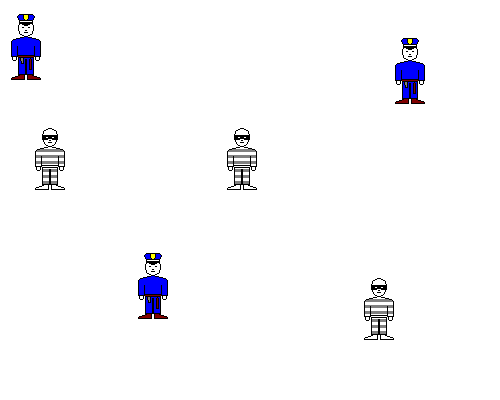
If the cops and robbers are widely spread out, they'll rarely meet. Similarly, if the calcium and sulfate are both exceedingly dilute, they won't form a precipitate. If you put Ca and SO4 in water in such small amounts that their concentrations don't multiply out to 2.4 x 10-5 or greater, gypsum won't form.
We can expect the number of arrests per unit time will be (cops/square mile)(robbers/square mile) = constant. The same thing is true of chemical reactions.
The solubility constant of gypsum is 2.4 x 10-5. That means if you have solid gypsum in equilibrium with water, the concentration of Ca (in moles per liter) times the concentration of SO4 (in moles per liter) equals 2.4 x 10-5. If it's less, the gypsum will dissolve.
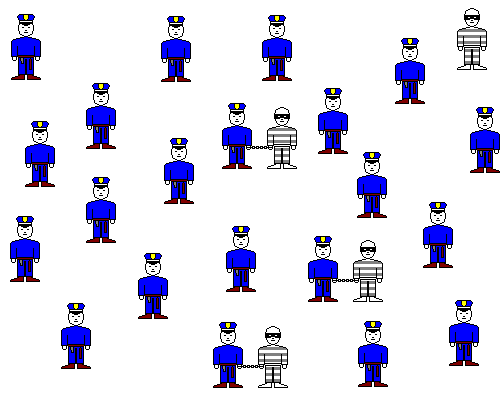
If there are far more cops than robbers, pretty soon almost all the robbers will be arrested, even if there are very few of them. If the sulfate concentration is extremely dilute, but the calcium is extremely concentrated, a precipitate will still form.
If there are far more robbers than cops, pretty soon almost all the cops will have arrested a robber, even if there are very few cops. If the calcium concentration is extremely dilute, but the sulfate is extremely concentrated, a precipitate will still form.
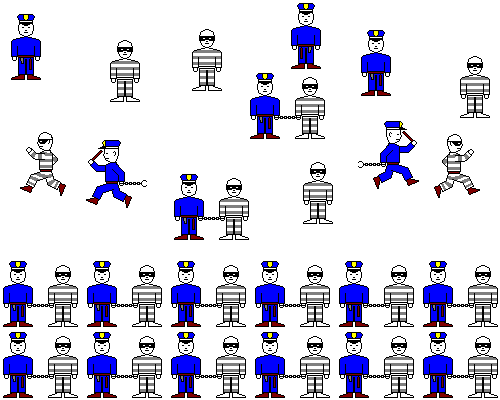
At equilibrium, we'll have cops booking robbers until the number of cops and robbers on the loose drops low enough for meetings to be infrequent. We'll still have occasional cops bringing in robbers, but we'll also have robbers escaping (or being sprung by lawyers, or serving their time). On the whole we'll have about the same rate of arrests as releases or escapes. The number of cops and robbers per square mile will stay about constant.
Similarly, if we put a chunk of gypsum in a liter of water, it will dissolve until (Ca)(SO4) = 2.4 x 10-5. If there's nothing else in the water, the concentrations of Ca and SO4 will be equal and we'll have (Ca) = (SO4) = √2.4 x 10-5 = .005. There will be .005 moles of Ca (.005 x 40 = 0.2 grams) and .005 moles of SO4 (.005 x (32 + 4*16) = 0.48 grams). As rocks go, this is very soluble. A cubic meter of water (1000 liters = 1000 kg) will dissolve 680 grams of gypsum.
If you're in a temperate rainy climate where it rains a meter per year, you'll dissolve away 680 grams or about 300 cubic centimeters per square meter per year. That will remove 0.03 cm/year from the surface, or 30 cm/1000 years, or 300 meters per million years. In geologic terms, that's carving with a chain saw.
 |
A gypsum pavement block from the Minoan site of Knossos, on Crete. In the century plus since the site was excavated it has undergone several centimeters of sulution. |
Sizes of Solubility Constants
Generally, the larger the solubility constant, the more soluble the material is. The smaller the solubility constant, the less soluble it is. For example, the solubility constant of NaCl is 3.6. For extremely insoluble galena (PbS) it's 10-29.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 03 April 2006, Last Update