Sphalerite and Chalcopyrite Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
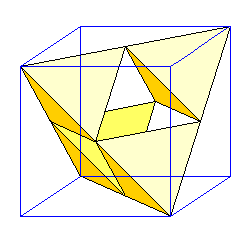 |
Sphalerite (ZnS) is one of a number of minerals whose structure is based on inscribing tetrahedra in a face-centered cube. |
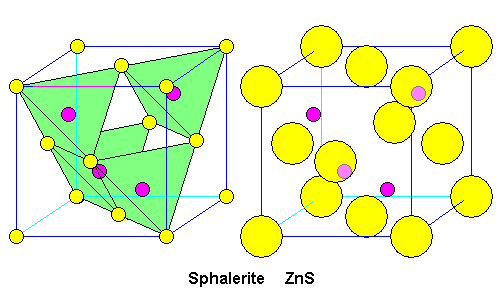 |
| In sphalerite, we have a face-centered cubic arrangement of sulfur (yellow) and zinc in the center of each tetrahedron (purple). At left is the structure illustrating the relationship to the tetrahedra. At right is the unit cell. Zinc atoms hidden behind sulfur atoms are shown in a lighter shade.
Is this really ZnS? We have four zinc atoms entirely within the unit cell. We have eight corner sulfur atoms, but they are shared among eight unit cells. There are six sulfur atoms in the centers of the faces, but they are each shared with a neighboring cell. So we have 8 x 1/8 + 6 x 1/2 = 4 sulfur atoms. There are an equal number of zincs and sulfur. |
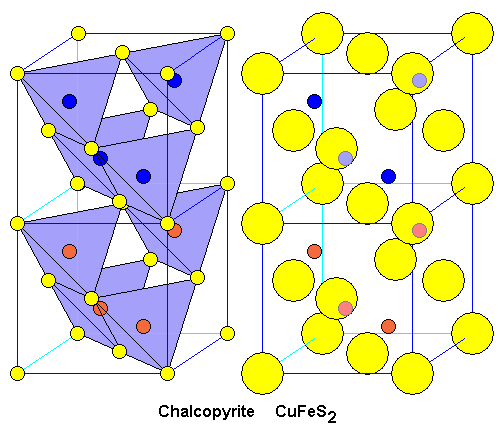 |
| Chalcopyrite has a very similar structure to sphalerite. Cells with iron atoms (orange) alternate with cells containing copper (blue). This double cell means chalcopyrite is tetragonal, but the fact that the unit cell consists of two cubes means chalcopyrite mimics cubic forms very often. In particular it can occur as pseudo-tetrahedra (actually tetragonal disphenoids, but they look exactly like tetrahedra.) |
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 February, 2001, Last Update