Sulfur Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
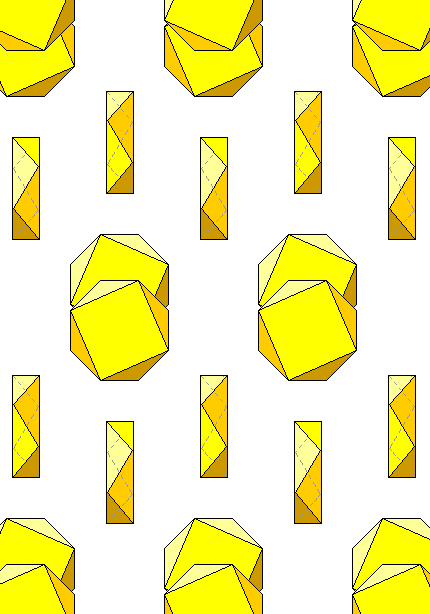
As elements go, sulfur has a bizarre structure. It has hybrid sp orbitals and bonds tetrahedrally (as we see in the sulfate anion). In the solid, it links to form kinked octagonal rings, although perhaps it's easier to see the structure by picturing its coordination polyhedra as flattened square antiprisms. The rings alternate in staggered rows, giving rise to the nickname "crankshaft" arrangement. The rings are held together by weak residual forces. The sharing of hybrid orbitals by sulfur, analogous to the way carbon atoms join, results in a wild variety - over 30 - of atomic structures, or allotropes, of sulfur, including other kinds of rings, chains, and the like. However, since sulfur doesn't have any other bonds available, it's not a potential xenobiological replacement for carbon.
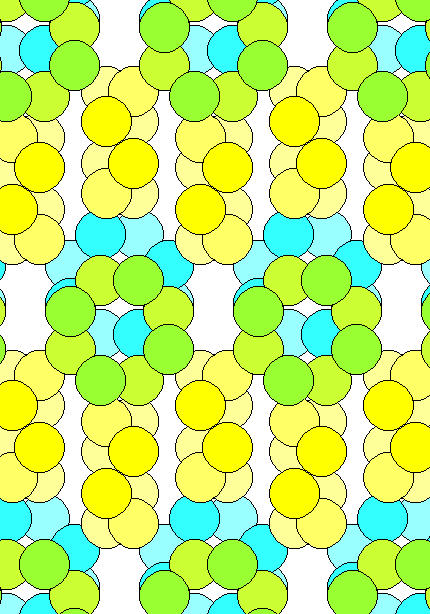
The diagram aboveshows the arrangement of atoms in solid sulfur. In a seriously non-traditional color scheme, the face-on rings are green in the foreground and blue in the background. Notice that there's a lot of interatomic space, so even though sulfur is a fairly heavy atom (atomic weight 32) the density of solid sulfur is only about 2.0 gm/cm3. Its covalent radius is 102 pm, its unit cell measures 1045 x 1285 x 2446 pm for a volume of 3.285 x 109 pm3 and there are 16 rings in a unit cell. So the total volume of the atoms in a sulfur unit cell is 16 x 8 x 4/3pi x 1023 = 0.569 x 109 pm3. Thus the fraction of space occupied by atoms is 0.569/3.285 = 17%, or 5/6 of the structure is interatomic space. The closest packing possible for uniform spheres is 74%.
If only a sixth of the volume is occupied by sulfur atoms and the density is 2.0 gm/cm3, it follows that individual sulfur atoms must have a density about six times larger, or about 12. The volume of a sulfur atom is 4/3pi x 1023 = 4.445 x 106 pm3. = 4.445 x 10-24 cm3. The mass of a sulfur atom is 32/6.02 x 1023 gm = 5.316 x 10-23 gm. So the density of a single atom is 5.316 x 10-23 gm/4.445 x 10-24 cm3 = 11.96 gm/cm3. This isn't a particularly meaningful calculation because the radius of an atom is fuzzy but it does underscore how fluffy the structure of sulfur is.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 15 October 2009, Last Update