What Atoms of the Heavy Elements Really "Look Like"
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Cesium (55) and Barium (56)
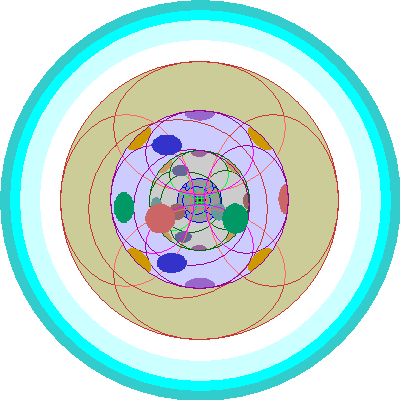 | Cesium (55) and Barium (56)
We add the 6s orbital. Cesium has one electron, barium two. The innermost shells (1-3) are completely full. Shell 4 has the full complement of s, p and d orbitals, shell 5 has s and p. |
The f Orbitals
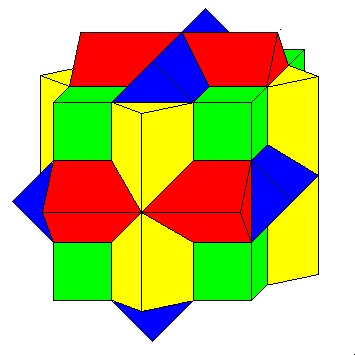 | The rare earth elements are the first ones to begin filling f orbitals. There are seven of them, and they are unusual because there are two representations in use. If the atom is in an environment with cubic symmetry, the best representation is a cubic one. Four orbitals are cubic in form, with lobes pointing along the diagonals of cubes. The arrangement is like the compound of four cubes shown at left.
The remaining three orbitals have two lobes and have their long axes along the axes of fourfold symmetry (where cube edges cross). The solid shown below has the symmetry of the overall set, which is called the cubic set. |
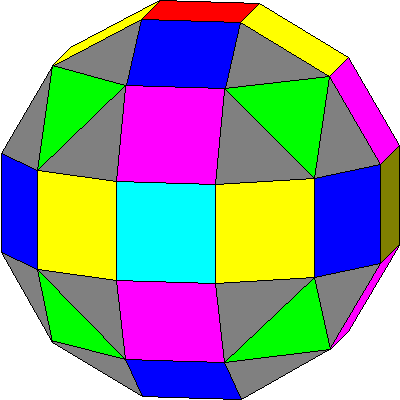 | In the diagram here, the four orbitals with cubic symmetry are green, magenta, yellow and dark blue. The three two-lobed orbitals are red, light blue, and brown. |
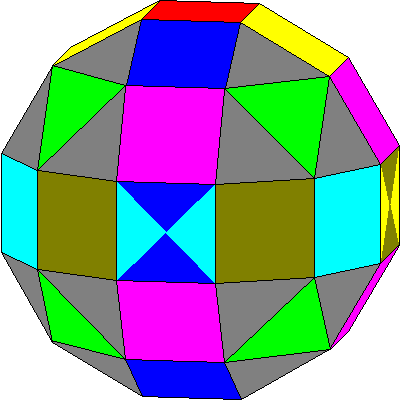 | The other set, called the general set, has the arrangement here. There is a polar two-lobed orbital (red), two orbitals with lobes in a cubic arrangement (green and magenta) and three mutually perpendicular planar six-lobed orbitals (light blue, dark blue, brown). Some of the lobes are coaxial, as suggested by the bicolored faces. |
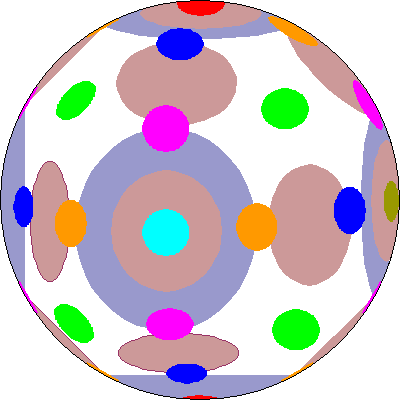 | Here's an attempt to show the p, d, and f orbitals all at once. P orbitals are largest and are dark gray, d orbitals are smaller and tan, and f orbitals are smallest and colored in accordance with the cubic set above. |
The Rare Earth Elements
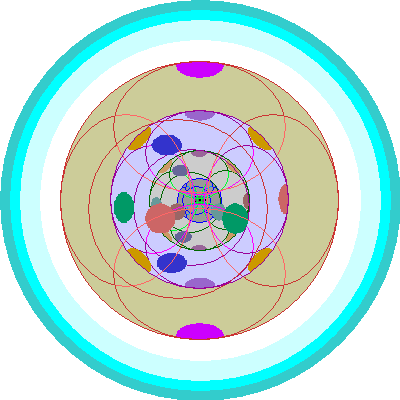 | Lanthanum (57)
Lanthanum adds an electron to a 5d orbital. Most of the remaining rare earth elements, however, add electrons by filling 4f orbitals. Since all the changes take place two shells in, even removing the 6s shell doesn't bring the 4f electrons to the surface. Therefore, all these elements are very similar chemically, since their outer shells, even as ions, are virtually identical. |
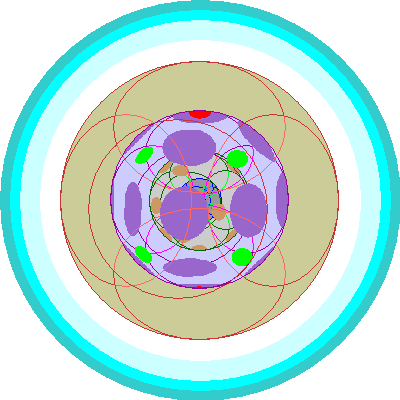 | Cerium (58)
Here we have to make yet another concession to clarity. We can no longer show the individual d orbitals in color. The 3d orbitals are tan and the 4d orbitals are dark purple. Cerium adds an electron in a 4f orbital (green). |
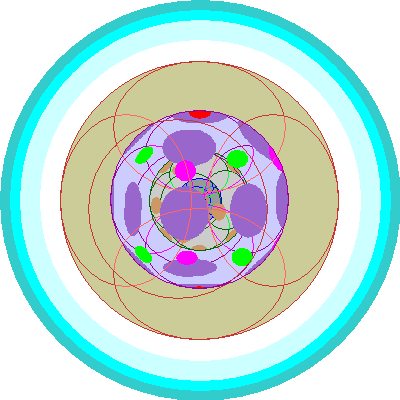 | Praseodymium (59)
Three 4f orbitals are occupied |
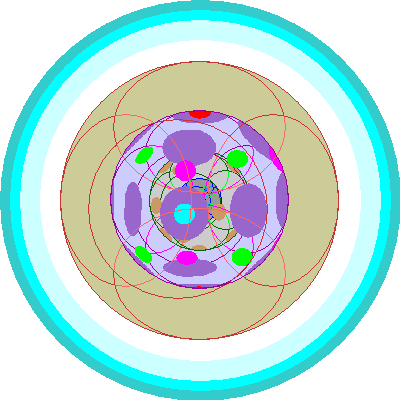 | Neodymium (60)
Four 4f orbitals are occupied |
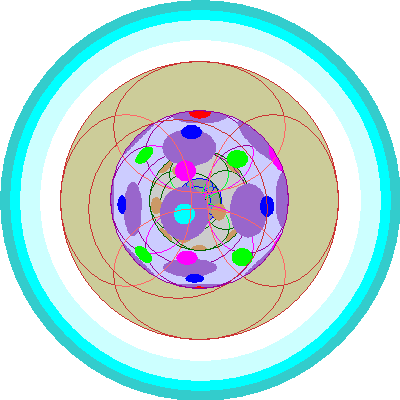 | Promethium (61)
Promethium is unstable but its electron configuration is perfectly orthodox, with five 4f orbitals occupied. |
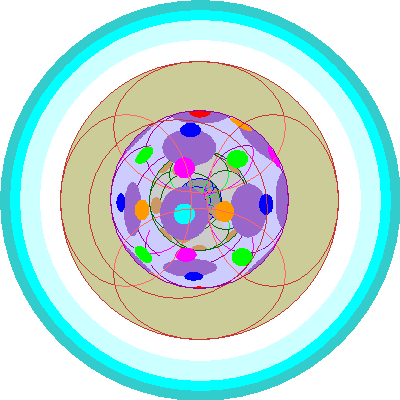 | Samarium (62)
Six of the seven 4f orbitals are occupied. |
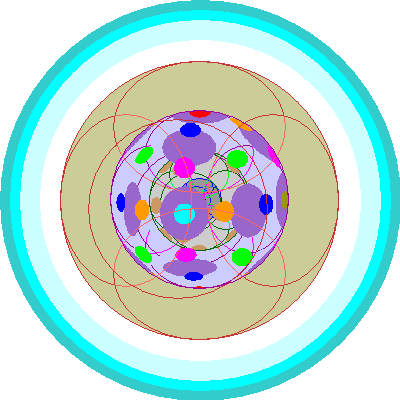 | Europium (63)
All of the seven 4f orbitals are occupied. |
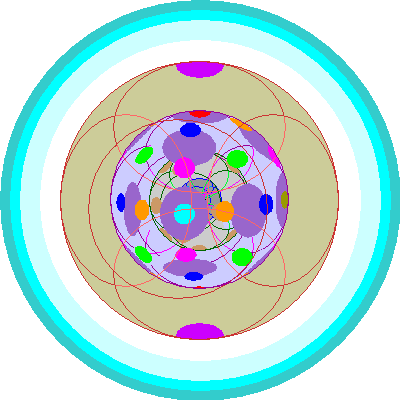 | Gadolinium (64)
All seven 4f orbitals are occupied. Gadolinium adds a 5d orbital rather than a second electron to a 4f orbital. |
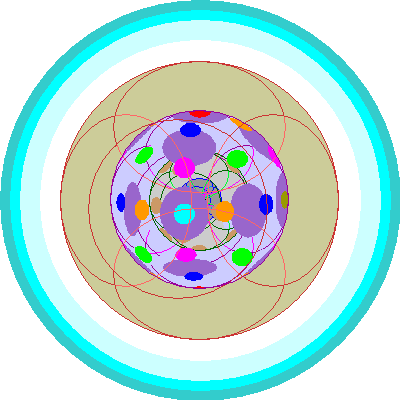 | Terbium (65), Dysprosium (66), Holmium (67), Erbium (68), Thulium (69), Ytterbium (70)
All seven 4f orbitals are occupied. Second electrons are added to each 4f orbital. |
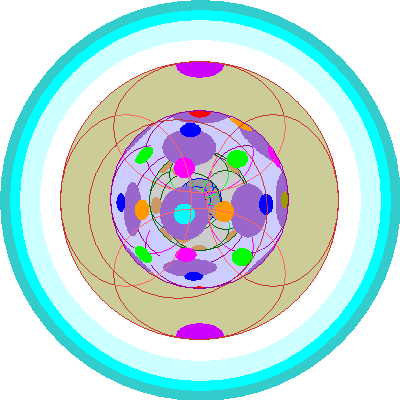 | Lutetium (71)
With Lutetium we begin adding 5d orbitals for keeps. Shell 4 is now completely full. |
Hafnium (72) Through Radon (86)
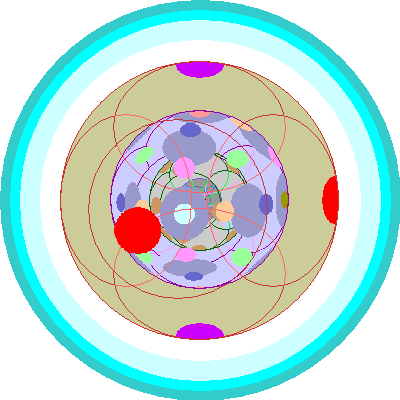 | Hafnium (72)
Hafnium (72), the first element after the lanthanides, adds a second 5d orbital. This is the Scandinavian neighborhood. Four elements (erbium, terbium, yttrium and ytterbium are named for the little Swedish town of Ytterby, Holmium is named for Stockholm and Hafnium for Copenhagen. Thulium is named for Thule, the ancient name for the far north. |
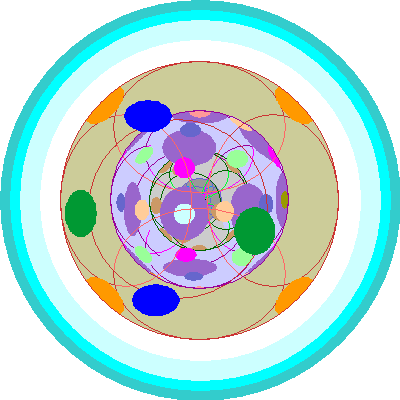 | Tantalum (73)
One electron goes into a third p orbital |
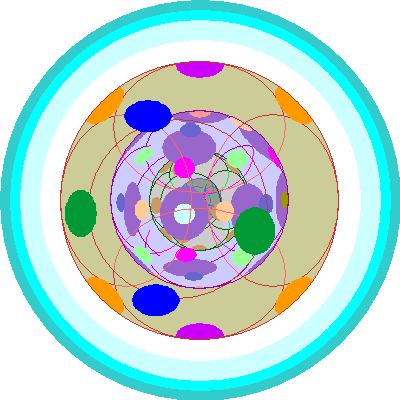 | Tungsten (74)
One electron goes into a fourth 5d orbital |
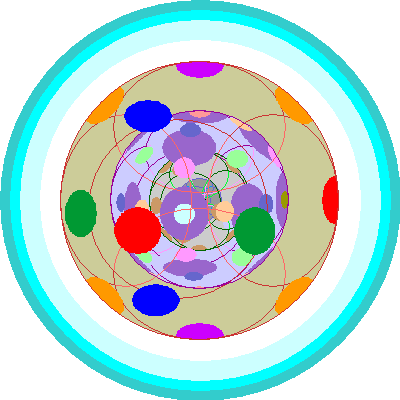 | Rhenium (75), Osmium (76), Iridium (77), Platinum (78), Gold (79), Mercury (80)
Now all five 5d orbitals are occupied. and with Osmium we begin adding a second electron to each. |
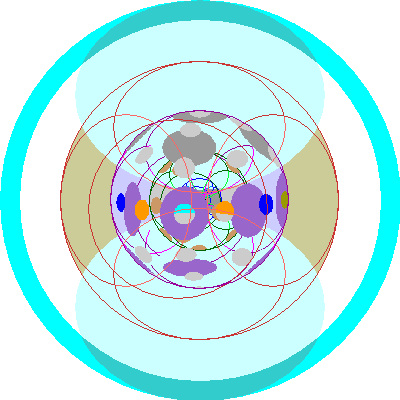 | Thallium (81)
We begin filling the 6p orbitals. |
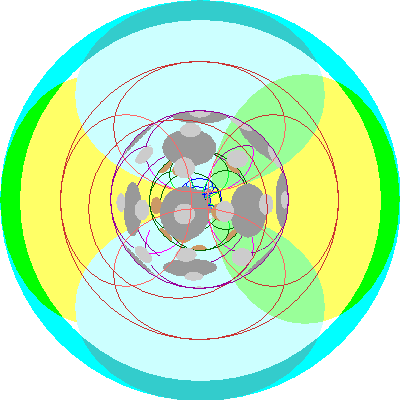 | Lead (82)
An electron is added to a second 6p orbital |
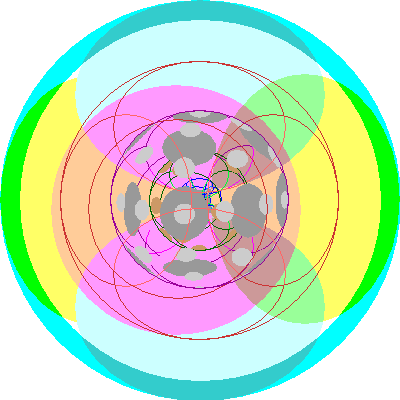 | Bismuth (83), Polonium (84), Astatine (85), Radon (86)
All three 6p orbitals are occupied and we add a second electron to each. Radon has a stable outer octet. Bismuth used to be considered the last element with a stable isotope, but it is actually radioactive with an extremely long half life, longer than the age of the universe. |
Francium (87) and Radium (88)
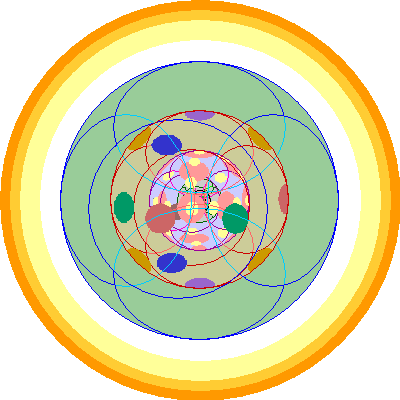 | We add a 7s orbital, which has one electron in Francium and two in Radium.
The 6p orbitals are in outline, the 5d orbitals in subdued colors and the 4f and 4d orbitals in pink and yellow. Details deeper within are all but impossible to see. |
The Actinides
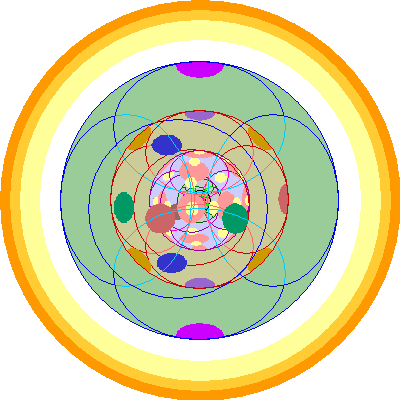 | Actinium (89)
Like Lanthanum, we begin by adding a p orbital, in this case 6p. |
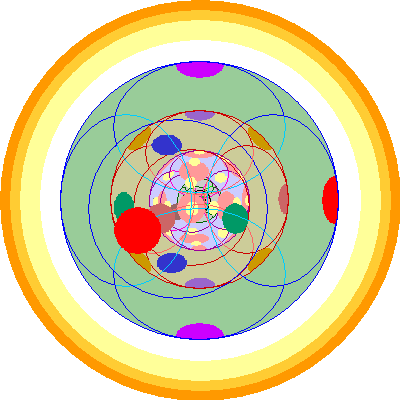 | Thorium (90)
We add a second 6p orbital. |
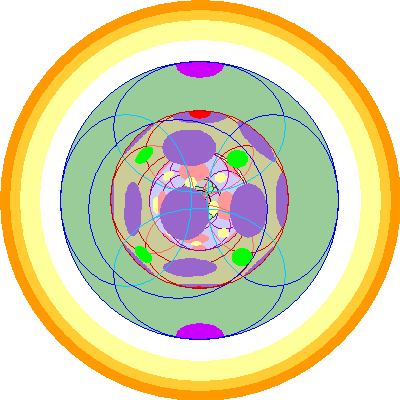 | Protactinium (91)
We lose one 6p orbital but add two 5f orbitals. The 5d orbitals are dark purple. |
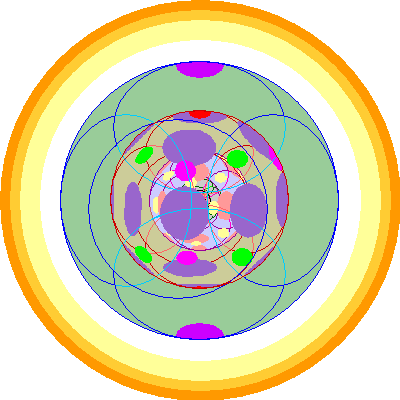 | Uranium (92)
We add a third 5f orbital |
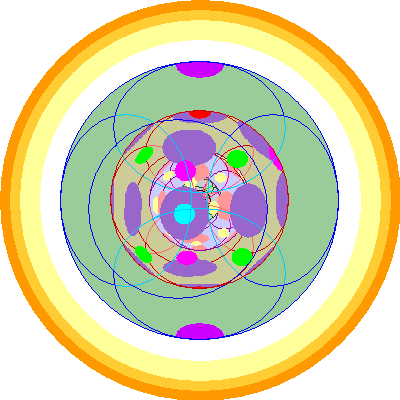 | Neptunium (93)
We add a fourth 5f orbital |
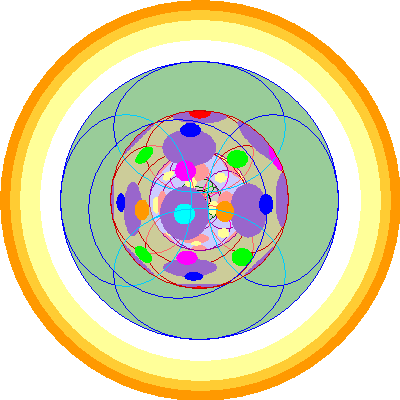 | Plutonium (94)
We lose the 6p orbital and add two 5f orbitals to bring the total to six.
|
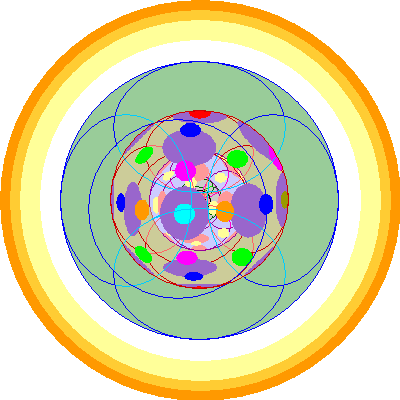 | Americium (95)
We add the seventh and final 5f orbital.
|
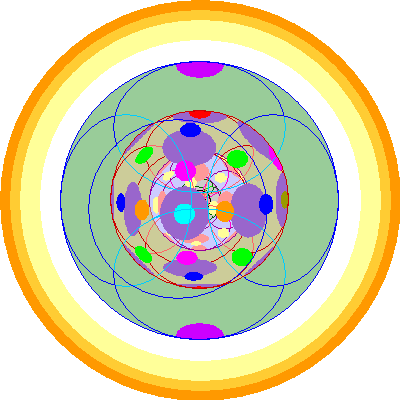 | Curium (96)
We add a 6d orbital.
|
 | Berkelium (97), Californium (98), Einsteinium (99), Fermium (100), Mendelevium (101), Nobelium (102)
The 5f orbitals are filled out by successively adding a second electron to each. |
 | Lawrencium (103)
Last of the actinides adds a 6d orbital.
|
At some point, we have to ask when the elements cease to exist in any meaningful sense. Plutonium-244 has a most respectable half life of 82.6 million years, and in fact a little primordial plutonium still exists on earth. Americium-243 has a half-life of 7380 years, and the isotope used in smoke detectors, Americium-241, has a half-life of 432.2 years. Einsteinium 254 has a half life of 276 days and Fermium 257 has a half life of 80 days. Lawrencium-262 has a half life of 4 hours, Dubnium-268 16 hours, Hassium-277 12 minutes and even Element 112 has an isotope of mass 285 with a half life of 10 minutes. Those could all potentially be created in amounts big enough to do actual chemistry with. The few atoms ever made beyond that have half-lives of seconds or less. In fairness to the guys at Berkeley, Dubna, and Darmstadt, the problem may be that we can't supply enough neutrons to make stable nuclei for very high atomic numbers.
An interesting feature is that the atoms don't get extremely large, due to the increasing attraction of the larger number of protons. But the mass increases, so ultra-heavy elements can be ultra-dense as well. Bohrium (107), Meitnerium (108) and Hassium (109) are the densest known elements with predicted densities of 37.1 g/cm3, 37.4g/cm3 and a whopping 41g/cm3 respectively. None of these elements have been produced in macroscopic amounts, so the densities are predicted theoretically. Hassium would be more than twice as dense as gold or uranium, and nearly twice as dense as osmium and iridium, the densest stable elements, which weigh in at a featherweight 22 g/cm3.
Somebody recently asked on a discussion site about making things out of bohrium. Apparently that was mentioned on some video game. The onbious answer is that it hasn't been made in macroscopic amounts. I also suspected the critical mass would be absurdly tiny. That turns out not to be the case. Critical mass depends on neutron capture cross section and even einsteinium (99), the heaviest element for which there's data, has a [predicted] critical mass of kilograms. Einsteinium is also the heaviest element made in macroscopic amounts. Since it has a critical mass of kilograms and it's only been made in milligram amounts, and then only sporadically, the likelihood of an einsteinium nuke is pretty slim.
What Do Atoms Really "Look Like?"
What Atoms of Hydrogen Through Xenon Really "Look Like"
What Atoms of the Heavy Elements Really "Look Like"
Scale Drawings of Atoms and Orbitals: Rubidium Through Xenon
Scale Drawings of Atoms and Orbitals: Cesium Through Radon
Scale Drawings of Atoms and Orbitals: Francium Through Lawrencium
What the Atomic Structures of Some Simple Materials Really "Look Like"
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 20 September 2005, Last Update
Not an official UW-Green Bay Site