What the Earth is Made Of
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
The Sun and Stars
The Sun is a pretty typical star whose composition is known from spectroscopy. The composition of the Sun in atomic abundance is shown below. Silicon is used as a basis for comparison because it allows convenient comparison of solar and planetary element abundances.
Note that the abundance scale is logarithmic
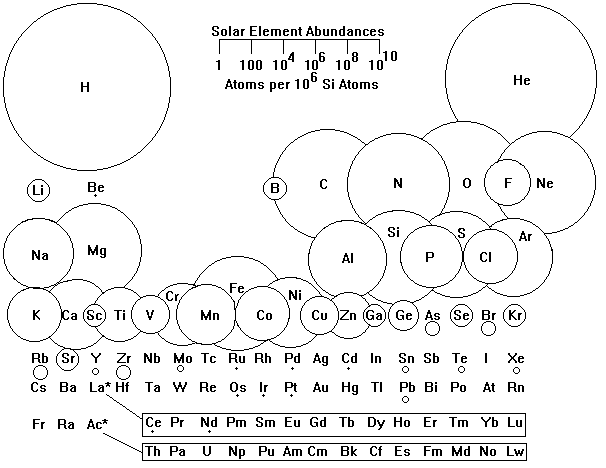
From the figure above, we see four patterns:
- An overwhelming abundance of light elements
- A strong preference for even-numbered elements
- A peak in abundance at iron, followed by a steady decrease.
- Elements 3-5, Lithium, Beryllium and Boron, are very low in abundance.
These patterns have to do with nucleosynthesis (element formation) in the stars
A normal star like the Sun converts hydrogen into helium by a series of proton-proton collisions followed by emission of particles. The Sun cannot make heavier elements. So where did the other elements come from? Earlier generations of stars. The Sun is probably a third-generation star.
(Incidentally, the Sun cannot make heavier elements than helium, but it can make use of those that are already there. One energy process in the Sun called the CNO cycle adds four protons to a carbon atom and results in eventual creation of a heavier atom that splits to yield a helium nucleus plus a carbon atom. In essence, the carbon is a nuclear catalyst for creating helium.)
From helium, there are two ways to get heavier nuclei. We could add particles one at a time. However, all nuclei of mass 5 are extremely unstable. Or we could collide helium atoms, except that all nuclei of mass 8 are also very unstable. At first glance it looks like stars cannot create elements heavier than helium. However, when massive stars run low on hydrogen, they begin to collapse under their own gravity. Eventually, their interiors become so hot and dense that three-way collisions of helium nuclei can occur. The result is a nucleus with six protons and six neutrons: carbon. In still more massive stars, helium nuclei (alpha particles) collide with existing nuclei to make oxygen, neon, and so on.
Thus
- The abundance of light nuclei is due to the increasing difficulty of building heavier nuclei in stars.
- The preference for even numbers is the result of successive collisions of alpha particles. Odd-numbered elements form by collisions of single particles with nuclei.
- The jump from helium to carbon explains the rarity of lithium, beryllium and boron. These nuclei form by spallation - knocking pieces off heavier nuclei - and tend also to be destroyed by nuclear reactions in stars.
In extremely massive stars there are shells of nuclear fusion where progressively heavier nuclei form. The end result of fusion is iron, the most stable nucleus. Iron nuclei cannot yield energy either by fusion or fission. So where do even heavier nuclei come from?
One way, the s-process (for slow) involves stray collisions between iron nuclei and other atomic particles. Obviously, the more particles added, the rarer the element will be.
The other process is the r-process (for rapid - and how!). A massive star with a shell structure and an iron core might seem capable of eventually becoming solid iron as the shells migrate outward. It might, except for one thing: at some point the core becomes so massive its gravity overcomes the electrical repulsion between nuclei and the core collapses to become a neutron star, effectively a giant atomic nucleus.
The fusion core of the former star was perhaps the size of the Earth. In milliseconds it collapses to 10 km in diameter but is still more massive than our Sun. Under its incredible gravity, the surrounding star matter comes screaming in, reaching up to a tenth of the speed of light. Several times the mass of the Sun slams into a neutron star at a tenth of the speed of light. The results are, to put it mildly, impressive. Nuclear reactions run rampant, building nuclei as heavy as plutonium and probably far beyond (we still find tiny traces of the plutonium). The incoming matter bounces off as a shock wave, which gets additional energy from nuclear reactions. Withi a few hours, the outer layers of the star are blasted off and we look straight into the thermonuclear core of the star. The star brightens by hundreds of millions of times to become a type II supernova. Much of the light is due to the decay of radioactive iron, nickel, and cobalt.
Thus
- The abundance of iron is due to its being the most stable atomic nucleus.
- The elements heavier than iron are built in massive old stars. The farther beyond iron they are, the rarer.
The Planets
After any large construction job, there are always scraps of building material lying about. If the Sun and Solar System formed from the same material, we would expect the raw material of the planets to match the composition of the Sun, minus those elements that would remain as gases. We find such a composition in a class of meteorites called chondrites, which are thought to be the most primitive remaining solar system material. Chondrites are considered the raw material of the inner Solar System and probably reflect the bulk composition of the Earth.
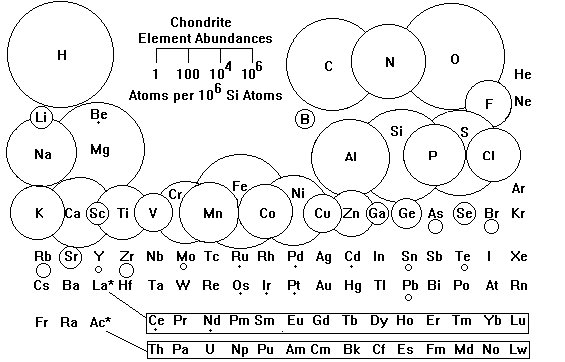
About halfway between Mars and Jupiter, it is cold enough for ice to persist even in a vacuum, so the moons of the outer planets are made of both rock and ice. On the outermost planets, methane, ammonia and finally nitrogen also condensed as solids. Comets are probably leftover raw material from the cold outermost Solar System, which is one reason they are so intensively studied.
Continental Crust
The continental crust of the Earth differs radically from the overall composition of the Earth.
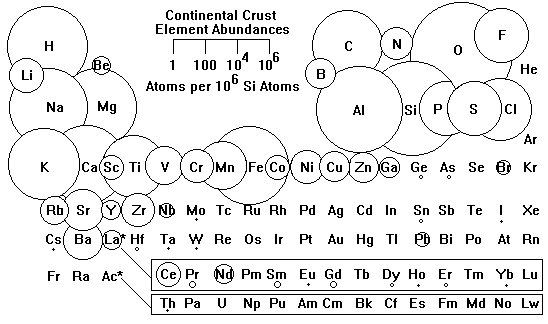
The elements across the middle of the Periodic Table, the Transition Elements, are very depleted relative to the earth as a whole, and certain elements very much enriched, notably the alkali metals, lead, uranium, and some rare earths. The transition elements are largely in the Earth's mantle and core. The enriched elements tend to be very large cations, especially those with large electrical charges.
This pattern reflects repeated partial melting of the mantle and accumulation of the residue in the crust. The mantle is mostly iron and magnesium silicates, ions with small sizes and electric charges of +2. Ions with large radii cannot fit mechanically very well into the mantle minerals and tend to leave at every opportunity. Ions with large electrical charges also require extensive substitution to be incorporated, and they also end up in the melt. Plate tectonics and repeated partial melting have created the granitic continental crust of the Earth.
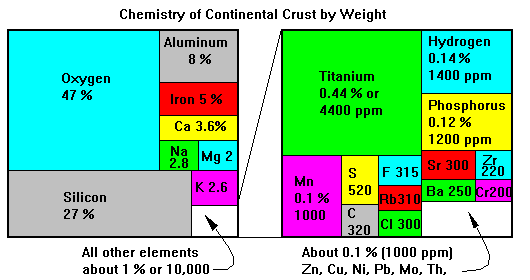
In weight terms, the more traditional way of accounting for element abundances, the continental crust looks like this. Silicon and oxygen account for three-quarters of the mass of the crust, and six more elements bring the total to 99 per cent. Note that many important metals, like copper, nickel, gold and silver, are missing even from the last one per cent diagram. We can mine these elements only because geologic processes concentrate them in the crust.
Note the difference between atomic and weight abundance. Hydrogen and iron are similar in atomic abundance, but iron, with about 50 times the atomic mass of hydrogen, is about 50 times more abundant by weight.
The Moon
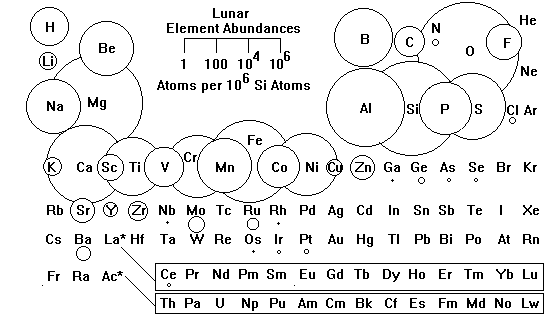
The Moon looks quite different from chondritic material and the continental crust. Hydrogen is enormously depleted, reflecting the almost total absence of water on the Moon. Among the metals, the right side of the Periodic Table is strongly depleted. These elements tend to be more volatile, and the Moon is strongly depleted in volatile materials. Generally, the lower the boiling point of an element, the lower its abundance on the Moon. This pattern suggests the Moon formed in a hotter region of the Solar System than did the Earth.
Return to Crustal Materials Notes
Return to Professor Dutch's home page
Created Sept. 4, 1997
Last Update Sept. 15, 1997
Not an official UW-Green Bay Site