Wells' "Hyperbolic" Tesselations
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Crystallographer A.F. Wells published a long series of papers in the late 1960's that described a number of hyperbolic tesselations.
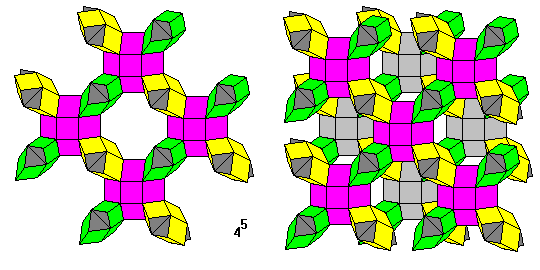
This lovely and intricate tesselation has five squares meeting at each vertex. It is formed from cross-sheped modules, each consisting of a cube and four triangular prisms. The modules lie in three orientations as shown. On the left is a single layer of the pattern, on the right are two layers with the rear layer colored gray.
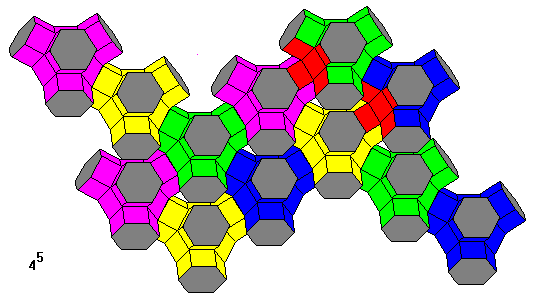
This pattern can also be considered as made of truncated octahedra joined by hexagonal prisms. This illustration shows the tesselation from that perspective. Two of the cross-shaped modules are colored red.
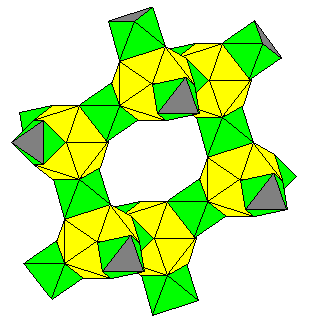 |
Icosahedra can be connected by octahedral tunnels to create a tiling with seven triangles meeting at each vertex. Shown here is a six-membered ring repeat unit. |
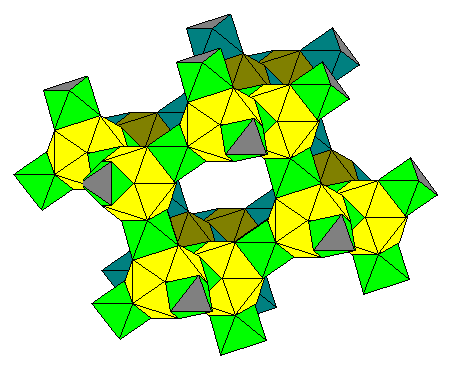 |
Six-membered rings can in turn be connected to form a three-dimensional structure. The icosahedra basically form a lattice identical to the geometric structure of diamond. The rear layer is shown here in darker tones. |
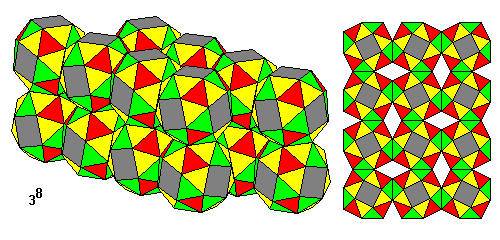 |
Snub cubes, alternately left- and right-handed, can be joined on their square faces to form a tesselation with eight triangles at each vertex. |
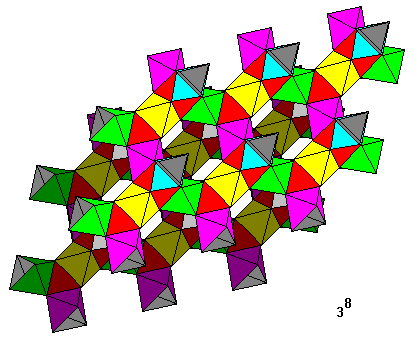 |
Here we have octahedra connected by octahedral tunnels. The linkages, like the tetrahedrally-linked icosahedra above, form the diamond crystal lattice. |
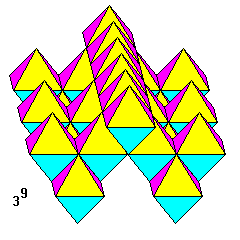 |
Nine triangles meet at a vertex. Rows of octahedra enclose tetrahedral voids. This is a geologically very important pattern: it's the atomic structure of spinel (MgAl2O4). The oxygen atoms are located at the octahedron vertices, which are in a cubic close-packed arrangement. The aluminum atoms are at the centers of the octahedra and the magnesium atoms at the centers of the tetrahedra. (This is the reverse of what we would normally expect based on the relative sizes and ionic charges of Mg and Al atoms.) |
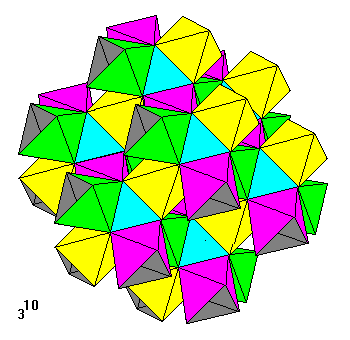 |
This tiling, ten triangles around a vertex, is built up of units consisting of a central octahedral void surrounded by a ring of six octahedra. Only a pair of opposite faces of the central octahedron are visible (shown here in light blue). Six units can link into a zigzag ring, as shown here, with another unit on top to show how layers join. |
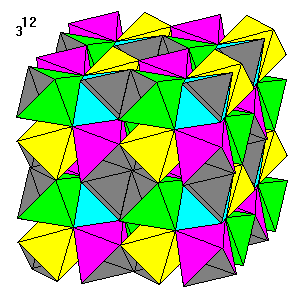 |
This seems to be the densest tiling known: twelve triangles meeting at a point. It can be considered as made up of units of eight octahedra surrounding a central octahedral hole. The eight octahedra form a truncated cube unit. The enclosing cubes are truncated 3/8 of the cube edge from each corner, and the recessed central vertices are 1/8 of the cube edge in from the center. By omitting one opposing pair of octahedra we obtain the basic unit of the preceding 10 x 3 tiling. |
Go to Symmetry and polyhedra Page
Go to Recreational Mathematics Index
Return to Professor Dutch's Home Page
Created 10 March 1999, Last Update 10 March 1999