Goethite and Diaspore Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Goethite, (FeO(OH)) and diaspore (AlO(OH)) are among the many hydroxides of iron and aluminum. Usually these minerals are so intermingled that we apply collective names to the mixtures: limonite for iron and bauxite for aluminum. In both cases, the oxygen-hydroxyl sheets form a hexagonal close-packed array. The iron and aluminum fill two thirds of the octahedral interstices, but in strips.
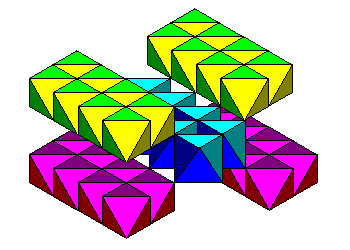 | An oblique view. In a polyhedral representation, strips of octahedra enclose "tunnels." |
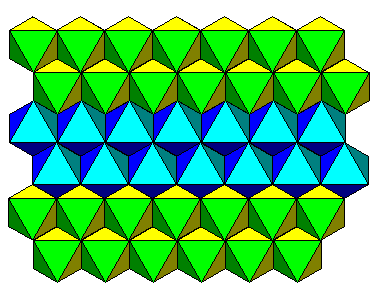 | Top view. Note that the octahedra at the two different levels face opposite directions. |
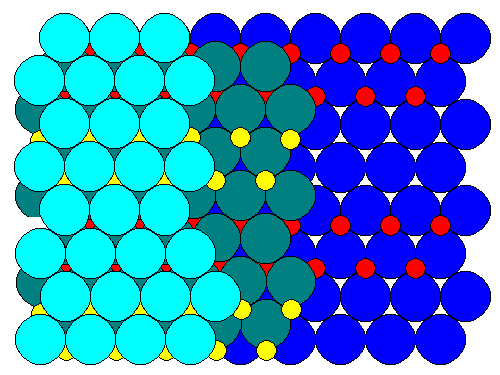
In the ball model below, we see that alternating strips of cations (red andyellow) join the close-packed oxygen sheets (in shades of blue). There are no"tunnels" - there appear to be in the polyhedral representation only because vacant octahedra are not shown.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 6 March, 2002, Last Update