Phyllosilicates
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
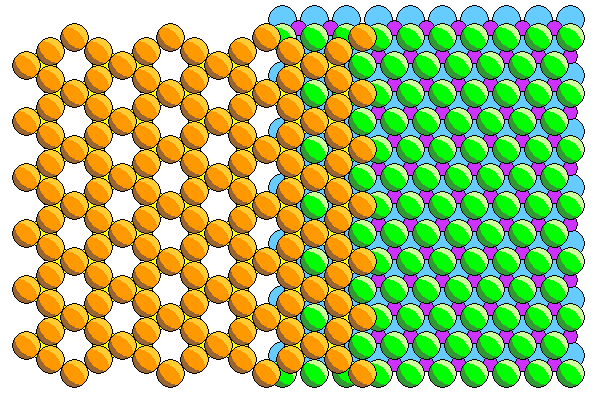
Phyllosilicates consist of sheets of silica tetrahedra attached to close-packed sheets of aluminum (gibbesite) or magnesium hydroxide (brucite). Most textbooks show neat hexagonal silica sheets like those above but in reality the arrangement is a lot more like that below shown in polyhedral form. The bases of the tetrahedra are shown with hidden edges in gray.
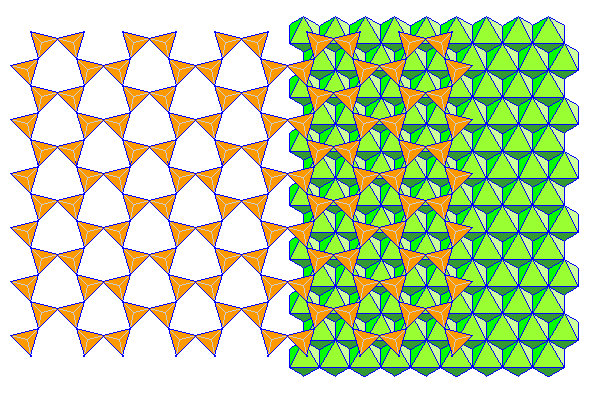
Reference for the modified silica sheet: Zoltai, Tibor; Stout, James H.; Mineralogy: Problems and solutions (ISBN: 9780808726104) Burgess, 1985.
Brucite and Gibbsite
Brucite and Gibbsite are hydroxides of magnesium and aluminum, respectively. They consist of close-packed sheets of hydroxyl ions with cations in the octahedral intersitices. In Brucite (Mg(OH)2) there are two hydroxyls for every magnesium atom so every interstice is filled. A trioctahedral brucite sheet is shown below in plan view and edge on.

In Gibbsite (Al(OH)3), shown below, the Al has a +3 charge so there are only 2/3 as many filled interstices as in Brucite. The filled interstices form a hexagonal network. What look like holes in the polyhedral representation are merely intersitices with no cation. The hydroxyls form two close-packed layers.

The names of these two configurations are a bit confusing. The Brucite sheet is called trioctahedral because 3/3 of the interstices are filled. The Gibbsite sheet is called dioctahedral because only 2/3 are filled. The di- and tri- prefixes do not refer to the charges on the cations! They refer to the fraction of filled intersitces.
The sheets are held to one another by weak residual forces, Van der Waals and hydrogen bonds. So both minerals are very soft and occur usually as microscopic crystals.
Antigorite and Kaolinite
Antigorite and Kaolinite are open-faced sandwiches consisting of a silica sheet bonded to a Brucite or Gibbsite sheet on one side. Antigorite contains the Brucite sheet and Kaolinite has the Gibbsite sheet. Below is an antigorite sheet seen edge on. In kaolinite, every third octahedral void is empty.

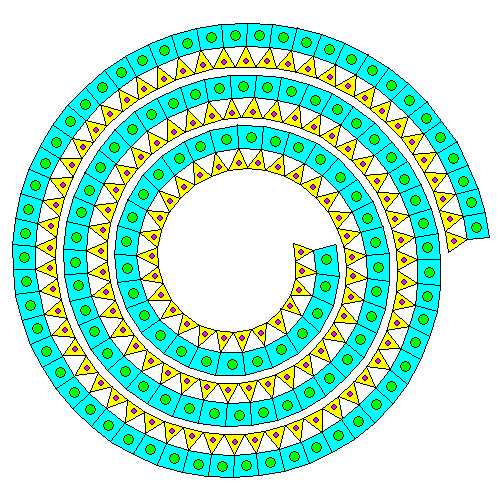
Mating a silica sheet and a brucite sheet results in deformation of both. Neither the silica sheet nor the brucite sheet especially like being strained. If there's only a silica sheet on one side, the mismatch causes the pair of sheets to curl into spirals and tubes. Thus, even though it's a phyllosilicate, the mineral ends up forming thin fibers. This is the serpentine form of asbestos. In the diagram above, silicon atoms are orange and silica tetrahedra are yellow. Magnesium atoms are blue and magnesium octahedra are green.
"Go ahead, deform my lattice. Just for that, I'll give the humans mesothelioma. Buwahahahaha." Actually, it appears that serpentine asbestos dissolves fairly quickly in the body and that amphibole asbestos is the real villain.
Talc and Pyrophyllite
Talc and Pyrophyllite are two-sided sandwiches. Talc consists of a Brucite sheet between two silica sheets, and Pyrophyllite has a Gibbsite sheet. Below is a Talc sheet seen edge on. In Pyrophyllite, every third octahedral void is empty.

Chlorite
Chlorite consists of alternating Talc and Brucite sheets as shown below.

Clay Minerals
Clay minerals fall into four groups, each based on the structure of one of the basic phyllosilicates.
| Phyllosilicate | Clay Mineral |
| Kaolinite | Kaolinite Group |
| Muscovite | Illite Group |
| Pyrophyllite | Montmorillonite (Smectite) Group |
| Talc, Chlorite | Vermiculite |
Vermiculite

Vermiculite has talc sheets but in addition it has ferromagnesian sheets with water molecules on either side. The alternation of talc and ferromagnesian sheets is similar to chlorite. The hydrogen atoms (red) bond the water molecules through hydrogen bonding and also bond the ferromagnesian and talc sheets through hydrogen bonding. On heating, the water vaporizes and expands the mineral, creating the worm-like stacks of sheets that give the mineral its name.
The Micas
The micas consist of Talc or Pyrophyllite sheets joined by other cations, most commonly potassium

- Biotite consists of Talc sheets with extensive substitution of iron for magnesium.
- Phlogopite also consists of talc sheets but with much less iron. It tends to be found in metamorphosed magnesium rich but iron poor rocks like dolomite.
- Muscovite consists of Pyrophyllite sheets.
- Paragonite is like muscovite but with sodium instead of potassium. Paragonite is uncommon. One hypothesis for its scarcity is that the smaller size of the sodium atom allows it to fit into many more mineral structures than the larger potassium ion. Thus potassium is restricted to minerals with very open atomic structures.
- Lepidolite is a lithium mica. The tiny lithium ion does not replace potassium, as one might suppose from its location in the Periodic Table, but fills the "pyrophyllite" sheet. Because lithium only has a +1 charge, it fills all the octahedral interstices.
Brittle Micas and Other Layer Silicates
If we replace potassium with calcium in the mica structure, the extra charge is balanced by replacing some silica with aluminum. Between the tighter bonding between sheets due to the larger cationic charge, and the imperfections in the silica sheets, these micas lose their elasticity and easy cleavage. Thus these micas are called brittle micas. Margarite, the calcium analog of muscovite, is most common.
Glauconite, a common accessory in marine sedimentary rocks, has a structure similar to muscovite but with a great deal of substitution. Ca and Na substitute for K, ferric and ferrous iron and magnesium substitute for aluminum in the trioctahedral sheet, and aluminum substitutes for silica. It is rarely crystalline and generally occurs as fine grains.

Stilpnomelane (above) is similar to biotite in appearance and chemistry, but tetrahedra in the silica sheet point in both directions, linking to the ferromagnesian sheets on both sides. Obviously it lacks biotite's easy cleavage.

Chloritoid (above) resembles the brittle micas in having alternating Brucite and Gibbsite layers joined by silica, but the silica tetrahedra are not connected, so it is actually a nesosilicate. Also it is unusual in that the tetrahedra share edges with the octahedral cells in the Brucite and Gibbsite layers. Generally, Pauling's Rules imply that coordination polyhedra of cations with high charge and low coordination number, like silicon, avoid sharing edges.
Palygorskite

The interesting clay mineral palygorskite displays features related to several other minerals. Like stilpnomelane, it has silica sheets with tetrahedra alternately pointing up and down. It is a true phyllosilicate in that the silica sheets are continuous. But it doesn't have continuous brucite sheets. Instead it has strips of brucite octahedra, so palygorskite has some structural relationship to chain silicates. The brucite strips are alternately two and three octahedra wide. The octahedral vertices not shared with silica tetrahedra have water molecules attached. The oxygens are blue and the hydrogens are red.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 08 April, 2005, Last Update