Zircon Structure
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Zircon (ZrSiO4) is the principal repository for zirconium. It has a fairly simple structure. It has a tetragonal unit cell with a = 6.6 Angstrom units and c = 6. Zircon is perhaps the most important tetragonal mineral. Zirconium atoms are at the corners of the unit cell, the center, and at altitudes of 1/4c and 3/4 c along the midlines of each vertical [001] face. Silica tetrahedra are centered at top and bottom center, the midpoints of each edge parallel to c, and at 3/4c and 1/4 c along the midlines of each vertical face.
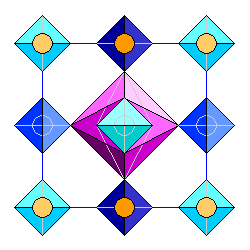 |
The zircon structure seen from the top, looking along the c axis. Silica tetrahedra are in blue, zirconium atoms in orange, with darker hues for increasing distance. The coordination polyhedron for the central zirconium atom is shown in purple. |
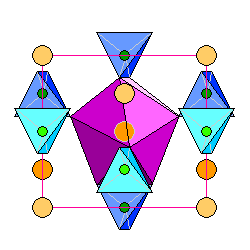 |
The zircon structure seen from the side, looking perpendicular to the c axis. Silica tetrahedra are in blue, zirconium atoms in orange, with darker hues for increasing distance. The silica tetrahedra are slightly skewed for perspective, but are really symmetrical about the faces. Silicon atoms are in green
.The coordination polyhedron for the central zirconium atom is shown in purple. The central zirconium atom, which is inside the purple polyhedron, is outlined in gray. |
The one somewhat odd feature of this structure is the coordination polyhedron of the zirconium atoms. The zirconium atoms are surrounded by eight oxygens, two sets of four at slightly different distances, but is not a slightly distorted cube. It is very nearly a shape called a snub disphenoid or Siamese dodecahedron. The ideal shape has 12 equilateral triangle faces and tetragonal disphenoid symmetry. One way to picture it as a very flat disphenoid cut into two pairs of faces and pulled apart along the symmetry axis, with a zigzag band of 8 triangles added in between. Another way is to imagine gluing two pentagonal pyramids together base to base along three of the five edges, then squeezing from the side and adding two more triangles to fill the opening.
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 26 February, 2001, Last Update
Not an official UW-Green Bay Site