Minerals
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Composition of Stars and Planets
Composition of the Crust
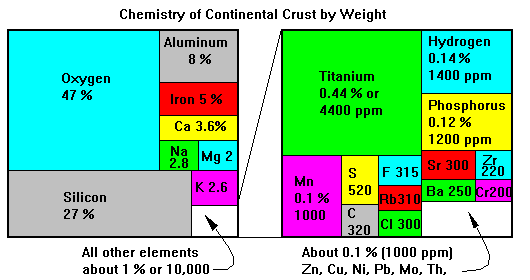
Eight elements make up 99 per cent of the crust. Note how rare many industrial metals are.
Minerals are the Chemicals that make up the Earth
- NATURALLY-OCCURRING
- INORGANIC
- CHEMICAL COMPOUNDS
- ABOUT 3000 KNOWN
- 200 COMMON
- 20 ROCK-FORMING
Atomic Bonding
1. IONS

Normal Configurations: Sodium: 11 p+, e-; Chlorine: 17 p+, e-
You may have heard that the pictures above are not really accurate. If you want to know more, you can visit
What Do Atoms Really "Look Like?" (optional)
ELECTRON SHELL STRUCTURE
Most stable arrangement: 8 e- in outermost shell
- Noble gases (Ar, Ne, etc.) have that arrangement naturally and rarely combine with anything else.
- Sodium: Loses electron, has 11p+, 10e-, charge +1: becomes a cation
- Chlorine: Gains electron, has 17p+, 18e-, charge -1: becomes an anion
- Many elements would have to gain or lose too many electrons and settle for other electron structures instead.
2. ELECTRICAL NEUTRALITY
(+) and (-) Cancel Out
3. BONDING (SATISFY 1 & 2)
- Ionic (NaCl)
- Covalent (O2)
- Metallic (Cu, Al, Fe)
- Hydrogen (in water)
Ionic and Covalent Bonding

- Ionic Bonding
- Some atoms gain electrons to become anions
- Others lose electrons to become cations
- Ions are attracted by their opposing charges
- Electrical Neutrality Maintained
- Most Important Bonding in Rocks and Minerals
- Covalent Bonding
- Electrons share electrons to fill incomplete shells
- Most Important Bonding in Organic Materials (and Organisms)
Metallic Bonding

A. Outermost electrons wander freely through metal. Metal consists of cations held together by negatively-charged electron "glue."
B. Free electrons can move rapidly in response to electric fields, hence metals are a good conductor of electricity.
C. Free electrons can transmit kinetic energy rapidly, hence metals are good conductors of heat.
D. The layers of atoms in metal are hard to pull apart because of the electrons holding them together, hence metals are tough. But individual atoms are not held to any other specific atoms, hence atoms slip easily past one another. Thus metals are ductile. Metallic Bonding is the basis of our industrial civilization.
Hydrogen Bonding

Hydrogen Bonding is Geologically Important
A. Water molecules are asymmetrical. The positively-charged portions of one are attracted to the negatively-charged parts of another. It takes a lot of energy to pull them apart. Hence:
- Water melts and boils at unusually high temperatures for such a light molecule.
- Water has a high heat capacity.
- It takes a lot of energy to melt ice and vaporize water.
- Thus water is the principal heat reservoir on the Earth.
B. The asymmetrical charge distribution on a water molecule makes it very effective in dissolving ionically-bonded materials. However, it is not an effective solvent of covalently bonded materials (oil and water don't mix). Hence:
- Water is very effective at weathering rocks and minerals. It is the closest thing to a universal solvent.
- Water is very effective at transporting ions and dissolved nutrients in the human body.
- Water is not an effective solvent of organic molecules. Thus we do not dissolve in our own cell fluids. Nifty feature.
C. When water freezes, it assumes a very open structure and actually expands. Most materials shrink when they freeze and sink in their liquid phases. Implications:
- If ice sank like most frozen solids, it would accumulate at the bottoms of frozen lakes and seas. Most of the world's water would be ice.
- Expansion of ice in rocks is a powerful weathering agent.
Summary of Bonding
- Ionic bonding holds rocks and minerals together
- Covalent bonding holds people and other organisms together
- Metallic bonding holds civilization together
- Hydrogen bonding gives water its heat-retaining and solvent properties
4. Lattice
Usually anions are bigger (They form framework and cations fill in spaces between). Thus it is often possible to remove one cation and replace it with another. Below, both halite (NaCl) and sylvite (KCl) have identical atomic structures and similar physical properties. They can be distinguished by their taste - sylvite is very bitter, somewhat like licking a belt sander. Those of you who have used so-called "light salt" know.
At right, a rubidium atom has substituted for potassium. Some elements, like rubidium, have no minerals of their own and occur in nature almost entirely by substituting for more common elements. In many minerals, this substitution occurs to such an extent that the mineral can be considered to consist of mixture of two or more ideal compounds. Such mixtures are called solid solutions.

5. Radicals
 |
Radicals are groups of atoms that behave as single units. Three of the most common are shown at left. |
Naming Minerals
Color
- Albite (Latin: Albus = White)
- Rhodonite (Greek: Rhodon = Rose)
- Glauconite (Greek: Glaucos = Blue-green)
- Azurite
Other Properties, Uses
- Magnetite
- Orthoclase (Straight + Break)
- Microcline (Small + Angle)
- Pyrophyllite (Fire + Leaf)
- Fluorite (Latin: Fluere - to Flow)
- Graphite (Greek: Graphos - Writing)
Components
- Magnesite
- Chromite
- Cuprite
- Siderite (Greek: Sideros - Iron)
- Calcite
Places
- Aragonite
- Muscovite (Moscow)
- Turquoise (Turkey)
- Andalusite
- Labradorite
People
- Scheelite
- Sillimanite
- Biotite
- Cordierite
- Wollastonite
Chemicals (And Minerals) Are Classified By Their Anions
For Example: Iron Compounds Have Little in Common
- Fe: Gray, Metallic
- FeCl2: Light Green, Water Soluble
- FeSO4: Light Green, Water Soluble
- FeCO3: Brown, Fizzes in Acid
- FeS2: Dense, Brittle, Metallic, Cubic Crystals
On the Other Hand, Sulfides have Many Properties in Common
- FeS2
- CuS2
- PbS2
- ZnS2
- All are Dense, Brittle, Metallic, have Cubic Crystals
Identifying Minerals
- COLOR -Sometimes Distinctive
- Often Unreliable
- Affected By
- Chemical Impurities
- Surface Coating
- Grain Size
- Weathering
- Hardness
- Resistance to Scratching
- Directly related to relative strength of atomic bonds
- Density
- Directly related to masses of component atoms and their spacing
- Usually very consistent
- Luster
- Metallic or Nonmetallic is the most important distinction.
- Resinous, waxy, silky, etc. are self-explanatory.
- Vitreous is often used for glassy luster.
- Cleavage
- Tendency to split along smooth planes between atoms in crystal
- Thus directly related to atomic structure
- Related to Crystal Form
- Every cleavage face is a possible crystal face
- Not every crystal face is a cleavage face. Quartz commonly forms crystals but lacks cleavage.
- Crystal form
- Takes Luck & Practice
- Well-formed crystals are uncommon
- Crystal Classification is somewhat subtle
- Fracture
- Geologic setting
- Some minerals occur in all geologic settings: quartz, feldspar, pyrite
- Some minerals occur mostly in sedimentary settings: calcite, dolomite
- Some minerals occur mostly in igneous settings: olivine
- Some minerals occur mostly in metamorphic settings: garnet, kyanite
- Special Properties
- Taste, Magnetism, Etc.
- Don't try on every mineral, but will quickly identify or rule out specific minerals.
- Experience And Reading
- Professional Methods
- Chemical Analysis
- X-Ray Studies
- Thin Section
Hardness
- Scratch Test (Mohs)
- Indentation Test (Knoop) - a more accurate scale used by metallurgists and engineers
Common Errors due to
- Weathering, Chalk' marks
- Breaking vs. Scratching
Mohs vs. Knoop Scales
- Talc: very small
- Gypsum, Fingernail: 30
- Calcite, Penny: 135
- Fluorite: 163
- Apatite: 430
- Feldspar, Glass: 560
- Quartz: 820
- Topaz: 1340
- Corundum: 2100
- Diamond: 7000
Density - gm/cm3
weight relative to water
Air: 0.001
Wood - Balsa: 0.1, Pine: 0.5, Oak: 0.6-0.9
Gasoline: 0.7, Motor Oil: 0.9
Ice: 0.92
Water: 1.00
Sugar: 1.59
Halite: 2.18
Quartz: 2.65
Most Major Minerals: 2.6-3.0
Aluminum: 2.7
Pyrite, Hematite, Magnetite: 5.0
Galena: 7.5
Iron: 7.9
Copper: 9
Lead: 11.4
Mercury: 13.6
Uranium: 19
Gold: 19.3
Platinum: 21.4
Iridium: 22.4 (densest material on Earth)
Major Mineral Suites
Elements
- Metallic:Au, Ag, Cu
- Not Al, Pb, Zn, Fe, etc.
- Nonmetallic: C - Diamond, Graphite
- Sulfur
Sulfides
Dense, Usually Metallic
Many Major Ores
- Pyrite FeS2
- Chalcopyrite CuFeS2
- Galena PbS2
- Sphalerite ZnS2
- Molybdenite MoS2
Halides
Usually Soft, Often Soluble
- Halite NaCl
- Fluorite CaF2
Sulfates
Soft, Light Color
- Gypsum CaSO4
- Barite BaSO4
Oxides
Often Variable, Some Ores
- Hematite Fe2O3
- Bauxite Al (OH)3 (a hydroxide)
- Corundum Al2O3 (Ruby, Sapphire)
Carbonates
Fizz in Acid, Give off CO2
- Calcite CaCO3
- Dolomite CaMg (CO3)2
Most Important Mineral Suite:
The Silicate Minerals
- Si + O = 75% of Crust
- Silicates make up 95% + of all Rocks
- SiO4: -4 charge
- Link Corner-To-Corner by Sharing Oxygen atoms
Nesosilicates - Isolated Tetrahedra
In the sketches, the O's represent oxygen atoms. The tetrahedra are viewed from above, and the Si atom would be below the central O atom. These are schematic only, the actual three-dimensional arrangement is more complex.
 |
Red circles denote other cations between the tetrahedra. The silica unit behaves like any other radical. The clue to a nesosilicate is SiO4 in the chemical formula. Representatives: -- Olivine -- Garnet -- Kyanite |
Sorosilicates - Paired Tetrahedra
 |
Epidote is the most common mineral of this type. The pair of tetrahedra has the formula Si2O7 |
Cyclosilicates - Rings
 |
Minerals with three, four, and six-sided rings are known. Examples of the rare three- and four-sided rings are at top. Six-sided rings are most common. The ring unit has the formula Si(x)O(3x) where x is the number of tetrahedra in the ring. Six sided rings thus have the formula Si6O18. The most common are: -- Beryl (Emerald - bottom left) -- Tourmaline (bottom right) |
Inosilicates - Chains
Single Chains
 |
The ratio of silicon to oxygen is 1:3, so these minerals have formulas with SiO3 or some multiple. Pyroxenes have this structure. Related minerals, called pyroxenoids, have single, but twisted, chains |
Double Chains
 |
These minerals, the amphiboles, have Si4O11 in their formula. A few triple-chain minerals are also known. |
Phyllosilicates - Sheets
 |
These minerals have Si2O5 in their formulas. The silica sheets are sandwiched with layers of magnesium and aluminum hydroxide, water, and other cations. There are many possible structures formed by the various layering possibilities but the main groups are: -- Micas -- Clay minerals -- Serpentine (asbestos) minerals |
Tectosilicates - Three-Dimensional Networks
These include Quartz and the Feldspars
 |
One of the simplest tectosilicate structures: tridymite, a high-temperature form of silica. |
 |
Quartz has a more complex structure, with spiral chains of tetrahedra. In this diagram they are colored differently to distinguish neighboring chains. |
 |
The structure of the feldspars. The red atoms are potassium, sodium, or calcium. Since these atoms are cations, some of the tetrahedra contain aluminum (+3) instead of silicon (+4) to maintain charge balance. |
The Problem of Crystals
Unit Cells
Repeating patterns, whether flowers on wallpaper, or atoms in a crystal, can all be described in terms of Unit Cells. A unit cell is an imaginary box that contains the basic pattern. Repeating the unit cells recreates the whole pattern. There are FIVE basic unit cells for two-dimensional patterns:
- Parallelogram (green)
- Rectangle (purple)
- Rhombus (yellow)
- Square (blue)
- Hexagonal (red)

Note that the rhombus pattern can be considered either as made up of rhombuses or as a rectangle with an extra point in the center. Crystallographers prefer the latter, because it makes the rectangular nature of the pattern clearer. Bricks in a wall have this pattern.
The hexagonal pattern can be described by rhombuses oriented in one of three ways. Two are in red and the third is outlined but not colored. The three unit cells lead to identical descriptions of the pattern.
Unit Cells in Three Dimensions

We can modify a cube by shaving off the edges, as shown in the top row. If we shave away the faces completely, the end result is a shape called a dodecahedron (Greek dodeka = twelve, hedron = side). The bottom left shows how cubic cells can be stacked to create this shape. The lower right shows the dodecahedron. In an actual crystal, the unit cells are so tiny the faces appear perfectly smooth.

We can modify a cube by shaving off the corners, as shown in the top row. If we shave away the corners completely, the end result is a shape called an octahedron (Greek okta = eight, hedron = side). The bottom left shows how cubic cells can be stacked to create this shape. The lower right shows the octahedron.
Below, we see some of the forms that can be made just from combining the three simple ways of stacking cubes above. Clearly, memorizing all the possible shapes is out of the question.
 |
Animation showing how the forms below are related |

Symmetry
 |
What crystallographers look for is the rules behind the shapes, called symmetry. All the shapes above can be cut in half in many ways to make mirror-image halves. This is called reflection symmetry.
They can also be rotated in various ways to positions where they look the same as their original orientation. This is called rotational symmetry. All together, there are 32 kinds of symmetry crystals can have, grouped into six classes according to the shapes of the unit cells in the crystal. |
 |
Animation of Symmetry
Upper Left: several possible ways of cutting the crystal into mirror-image halves are shown. There are numerous others not shown. Upper Right: Note that the crystal looks the same four times during a complete rotation. We refer to this rotation axis as a four-fold symmetry axis. Lower Left: The crystal looks the same three times during a complete rotation. We refer to this rotation axis as a three-fold symmetry axis. Lower Right: The crystal looks the same twice during a complete rotation. We refer to this rotation axis as a two-fold symmetry axis. |
Note (for the passionately interested only) that something irregular with no symmetry will only look the same once during a 360-degree rotation. Thus crystallographers say something with no symmetry has one-fold symmetry. It sounds convoluted, but all the mathematical formulas (yes, there is math in geology!) that are used to describe symmetry work perfectly.
The Crystal Classes
Just as plane patterns can be described in terms of five unit cells, three dimensional patterns can be thought of as belonging to one of six classes. Just as there are two kinds of rectangular plane patterns, there are several types of three-dimensional pattern for each of the six crystal classes
 |
Isometric or Cubic All edges equal, all angles 90 degrees Halite, Fluorite, Pyrite Galena, Garnet, Magnetite Gold, Copper, Diamond |
 |
Tetragonal Two edges equal, all angles 90 degrees. Square cross-section but different third dimension. Zircon Chalcopyrite |
 |
Orthorhombic No edges equal, all angles 90 degrees. Like the shape of a cereal carton. Olivine, Andalusite, Sillimanite Some Amphiboles and Pyroxenes Topaz, Sulfur |
 |
Monoclinic No edges equal, two angles 90 degrees. The shape obtained by knocking the ends out of a carton and skewing it. Some Amphiboles and Pyroxenes Micas Gypsum, Epidote Sugar also belongs to this crystal class. |
 |
Triclinic No edges equal, no angles 90 degrees Most Feldspars Kyanite Clay Minerals What if you have one 90 degree angle, or two equal edges? It turns out that these contribute no extra symmetry and the crystal is still triclinic. |
 |
Hexagonal Angles of 60, 90, and 120 degrees. Ice (snowflakes) Quartz, Beryl Corundum, Hematite Calcite, Dolomite |
Return to Earth Science Notes Index
Return to Physical Geology Notes Index
Return to Professor Dutch's home page
Created 8 February, 1997
Last Update 23 January, 2001
Not an official UW-Green Bay site