What Atoms of the Elements Really "Look Like"
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, Universityof Wisconsin - Green Bay
Lots of textbooks try to explain the real nature of electrons, and show sketches of the orbitals, and there are Web sites that give some very detailed pictures of orbitals, but generally they depict orbitals piecemeal. There don't seem to be any texts or Web sites that try to show what atoms with all their orbitals are like. So this is an attempt to do so. Maybe when someone invents a Holodeck, it will be possible to show atoms in exact detail, with the inherent fuzziness of orbitals and the true relative sizes and shapes of orbitals and all their nodal surfaces. Until then, we will have to make do with two-dimensional representations and we will quickly be forced to sacrifice detail for clarity as atoms get more complex.
Also, the radii of the orbitals are different within each shell. For this set of illustrations we will ignore the differences. The differences in orbital radii are small compared to the differences in shell radii. Bohr's model of the atom held that the radii of the shells were proportional to the square of their shell number, and that proportion is followed here, although in real atoms it's not perfectly true.
Orbitals don't fill in shell order. Instead some outer shells start acquiring electrons before inner ones do. The rule is shown below:
| 1s (h-he) | |||
| 2s (li-be) | 2p (b-ne) | ||
| 3s (na-mg) | 3p (al-ar) | 3d (sc-cu) | |
| 4s (k-ca) | 4p (ga-kr) | 4d (y-cd) | 4f (ce-lu) |
| 5s (rb-sr) | 5p (in-xe) | 5d (la)*(hf-hg) | 5f (th-lr) |
| 6s (cs-ba) | 6p (tl-rn) | 6d (ac)*(rf-112) | 6f |
| 7s (fr-ra) | 7p (113-118) | 7d | 7f |
The orbitals fill from top to bottom and right to left, down the colored diagonals. There's a slight exception at Lanthanum and Actinium. At Lanthanum, an electron goes into the 5d orbital, then the 4f orbital fills, then we continue filling the 5d orbital starting at Hafnium. A similar pattern holds for elements beyond actinium. For the larger orbitals, energy levels are so similar that orbitals no longer fill in strict sequence.
Elements 119 and 120 should fill an 8s orbital and at 121 we should expect a new kind of orbital (5g) to appear, but these atoms haven't been synthesized yet.
Hydrogen (1) Through Calcium (20)
 | hydrogen (1) and helium (2)
these have only one electron shell with a single orbital (1s) hydrogen has one electron, helium has two. |
 | Lithium (3) and Beryllium (4)
These have two electron shells. The first shell, which can only have two electrons, is full. The second shell has a single orbital (2s). Lithium has one electron in the 2s orbital, beryllium has two. |
 | Boron (5)
Boron's 2s orbital is full, so it has a 2p orbital as well. |
 | Carbon (6)
Since electrons tend to spread out among orbitals if they can, carbon adds a second p orbital with one electron in each. |
 | Carbon (6)
Sometimes, electrons in different orbitals are so similar in energy that the distinction between the orbitals disappears and the orbitals blur together or hybridize. Carbon is a common example. Its two 2s electrons and the two 2p electrons occupy four sp2 orbitals. Electrostatic repulsion pushes the orbitals apart into a tetrahedral arrangement. This accounts for the well known tetrahedral bonding of carbon. |
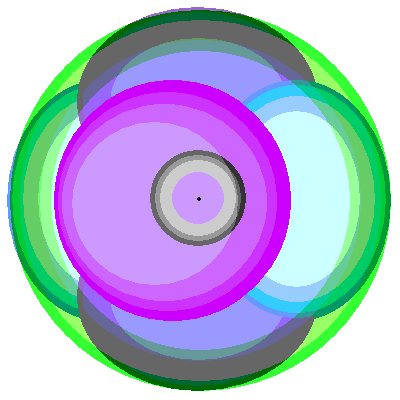 | Nitrogen (7), Oxygen (8), Flourine (9) and Neon (10)
Following the pattern of electrons spreading out if suitable orbitals are available, nitrogen adds a third p orbital. All the possible p orbitals are occupied, so the remaining elements add electrons to the existing p orbitals. Neon is the first element to have the complete stable outer octet of electrons. |
 | Sodium (11) and Magnesium (12)
The first and second shells are full and a third shell is added. For these elements the shell consists solely of a 3s orbital. Sodium has one electron in its 3s orbital, magnesium has two. |
 | At this point the complexity has reached the point where we'll have to adopt a still more schematic way of representing things. So we'll eliminate shading for the outer orbitals and show inner orbitals only in outline form. |
 | Aluminum (13)
Aluminum has a full 3s shell so it adds a 3p orbital. |
 | Silicon (14)
Silicon adds a second p orbital |
 |
Silicon (14) Like carbon, silicon has the hybrid sp2 orbitals which form tetrahedral lobes. Like carbon, silicon bonds tetrahedrally. There is no detailed information on the shapes of inner orbitals for any atom but there's no reason to doubt that the second shell of silicon is also hybridized. |
 | Phosphorus (15), Sulfur (16), Chlorine (17), Argon (18)
Following the pattern of electrons spreading out if suitable orbitals are available, phosphorus adds a third p orbital. All the possible p orbitals are occupied, so the remaining elements add electrons to the existing p orbitals. Argon, like neon, has a complete stable outer octet of electrons.
|
 | Potassium (19) and Calcium (20)
The two inner shells are complete and the p orbitals of the third shell are full. We add a fourth shell, beginning with an s orbital. Potassium has one electron in the 4s orbital, calcium has two. |
The Transition Metals
 | Here's where it gets to be fun. before adding 4p orbitals, we start adding 3d orbitals. Showing the d orbitals completely is impossible, so we will use dots to represent the outer ends of the lobes.
Because the 3d and 4s orbitals are so similar in energy, the addition of electrons is uneven, with electrons being added at times to the 3d orbitals and at times removed from or added to the 4s orbital. Scandium (21) Scandium has two electrons in the 4s orbital and one in the 3p. |
 | Titanium (22)
has two electrons in the 4s orbital and two in the 3p orbitals. There doesn't seem to be any generally accepted order for orbitals to be added, so in these diagrams they will be added in such a way as to maintain the most symmetrical arrangement. |
 | Vanadium (23)
has two electrons in the 4s orbital and three in the 3p orbitals. |
| The elements chromium through zinc (24-30) have five electrons or more in the d orbitals, and given the tendency for electrons to spread out, occupy all five orbitals. We skip past the option of having four orbitals occupied, probably because there's no really symmetrical way to do it and it's therefore energetically unfavorable. | |
 | Chromium (24)
Drops to one electron in 4s and five in 3d. Manganese (25), Iron (26), Cobalt (27, Nickel (28) All have two electrons in 4s and 5, 6, 7, or 8, respectively, in 3p. Copper (29), Zinc (30) All five d orbitals are occupied with two electrons. Copper has one 4s electron, zinc has two. |
Gallium (31) Through Xenon (54)
 | Gallium (31)
One 4p orbital with one electron. |
 | Germanium (32)
Two 4p orbitals with one electron each. |
 | Arsenic (33), Selenium (34)Bromine (35), Krypton (36)
Following the pattern of electrons spreading out if suitable orbitals are available, arsenic adds a third p orbital. All the possible p orbitals are occupied, so the remaining elements add electrons to the existing p orbitals. Krypton has a complete stable outer octet of electrons. |
 | Rubidium (37), Strontium (38)
The three inner shells are complete and the p orbitals of the fourth shell are full. We add a fourth shell, beginning with an s orbital. Rubidium has one electron in the 4s orbital, Strontium has two. |
 | Yttrium (39)
The 5s orbital has two electrons and there is one electron in a 4d orbital Yttrium is the first element in the second row of transition metals and from here to cadmium the pattern is very similar, though not identical, to the first set of transition metals. |
 | Zirconium (40)
The 5s orbital has two electrons and there is one electron in each of two 4d orbitals |
 | Niobium (41)
The 5s orbital has two electrons and there is one electron in each of four 4d orbitals |
 | Molybdenum (42)
The 5s orbital has two electrons and there is one electron in all five 4d orbitals Technetium (43), Ruthenium (44), Rhodium (45)The 5s orbital has one electron and there are 6, 7, or 8 electrons in the 4d orbitals, respectively. In terms of electrons, technetium is a perfectly respectable element. Its nucleus is unstable but its electron shells are just fine. |
 | Palladium (46)
A unique situation. Two electrons are added to the 4d orbitals while the 5s orbital is vacant. |
 | Silver (47), Cadmium (48)
The 4d orbitals are full. Silver has one electron in the 5s shell, cadmium has two. |
 | Indium (49)
Now we start filling 5p orbitals. There is one electron in a 5p orbital. |
 | Tin (50)
Two 5p orbitals, each with one electron. |
 | Antimony (51), Tellurium (52), Iodine (53), Xenon (54)
All three 5p orbitals are occupied, with 3, 4, 5, and 6 electrons, respectively. Xenon has a complete outer octet. |
What Do Atoms Really "Look Like?"
What Atoms of Hydrogen Through Xenon Really "Look Like"
What Atoms of the Heavy Elements Really "Look Like"
Scale Drawings of Atoms and Orbitals: Hydrogen Through Krypton
Scale Drawings of Atoms and Orbitals: Rubidium Through Xenon
Scale Drawings of Atoms and Orbitals: Cesium Through Radon
Scale Drawings of Atoms and Orbitals: Francium Through Lawrencium
What the Atomic Structures of Some Simple Materials Really "Look Like"
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 20 September 2005, Last Update
Not an official UW-Green Bay Site