Atoms and Light
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Kurt Nassau poses the question in The Physics and Chemistry of Color, why so many phenomena are crowded into the tiny band (less than an octave wide) of visible light. He responds that lower frequencies are dominated by molecular vibrations and are chiefly in the infrared, while ultraviolet frequencies are so energetic they strip electrons off atoms and can destroy atomic bonds. Visible light is the borderland where photons can just begin to interact with electrons.
But it goes deeper than that. The only reason we can see at all is because we have molecules in our eyes that can respond to visible light. And planetary atmospheres, be they oxygen-nitrogen, methane or carbon dioxide, are transparent to this narrow band. When the Russians landed probes on Venus, or the ESA dropped the Huygens lander on Titan, one thing they did not have to worry about was seeing, because those atmospheres are transparent to visible light. They would block infrared or ultraviolet, but visible light was transmitted.
So the fact that visible light occupies the narrow gap between molecular vibrations and ultraviolet absorption is what makes visible light visible. It's where electromagnetic radiotion just begins to interact with atoms, meaning planetary atmospheres are still transparent, but molecules (like in our retinas) can interact with light.
 |
As most people know, light is made up of waves, ranging from 700 nanometers at the red end to 400 at the violet end. Beyond those limits are many other kinds of electromagnetic radiation, not visible to they eye but detectable by their physical effects and by instruments. |
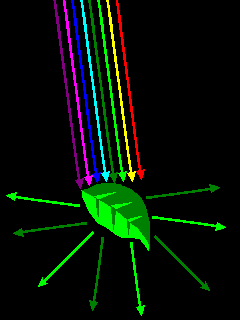 |
Materials have color because they interact selectively with visible light. Chlorophyll reflects green light but absorbs other wavelengths. |
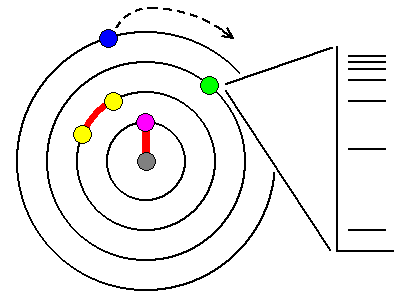 |
In
most atoms, the innermost electrons are bound so tightly to the
nucleus that visible light can't affect them (magenta). Only X-rays are
powerful enough, and in fact, exciting these electrons is a
principal way we makeX-rays. Most other electrons are paired (yellow)and too tightly bound for visible light to affect them. The outermost electrons (blue) are loosely bound and lost by ionization or atomic bonding. So that leaves elements with unpaired electrons in one of the outer shells (green). The energy levels available to these electrons are quantized and can only assume certain values, shown schematically at right. |
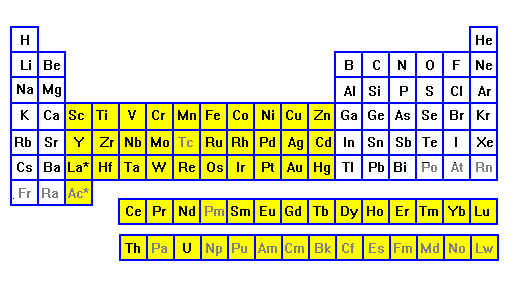 |
| Elements from lithium to nitrogen, and sodium through
phosphorus, give up their outer electrons in bonding, and their
inner electrons are complete octet shells. So none of these
elements produce color. Oxygen and fluorine, and sulfur and
chlorine, bond by filling their outer shells, and again they
don't result in color. It's in the next row that electrons start adding to the inner shells. These elements (yellow) can lose their outer electrons and still have unpaired electrons available to interact with visible light. These are the transition metals. |
 |
Of the most abundantr elements, O, Si, Al, Na, K, Mg and Ca do not cause color. Only Fe has unpaired electrons, and it is by far the most important source of color in rocks, minerals and soils. |
 |
The process of photons exciting electrons might be likened to a
pinball game. Just as a photon has to have just the right energy to
excite an electron, the pinball plunger and flippers have to impart
just the right amount of energy for the ball to hit a target. |
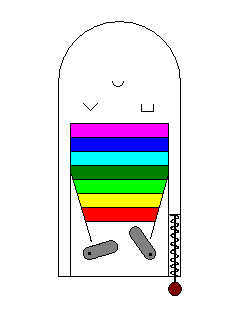 |
In the example here, there are no targets in the visible spectrum so no visible light photons will excite an electron and the material is colorless. |
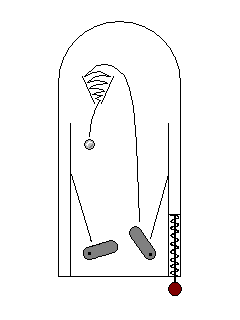 |
In some cases, an electron can be excited to a higher energy level, but take time to drop back to its initial state. This delayed light emission, familiar from glow-in-the-dark plastics, is called phosphorescence. |
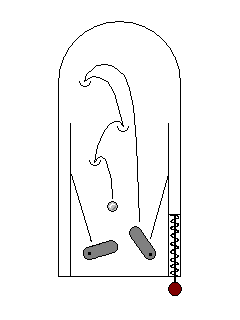 |
In some cases, an electron may be exicted by a high energy ultraviolet photon outside the visible range, but drop back to its original energy state in several lower-energy drops within the visible range. This phenomenon is called fluorescence. |
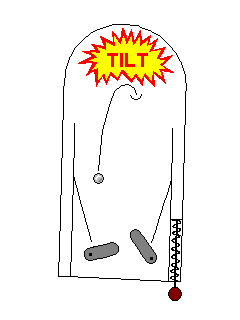 |
Sometimes an electron can be excited to a high energy level, but
drop back down to a lower level because of a physical disturbance.
If the disturbance is mechanical, like hitting, scratching, or
breaking, the effect is called triboluminescence. A common
example is the fact that some hard candies emit flashes of light
when bitten in the dark. If it's due to mild heating (far less than enough to make the material red hot) the effect is called thermoluminescence. |
Light, Wavelength and Energy
| Color | Wavelength, Angstrom Units | Wavelength, nanometers | Frequency (Hz) x 1014 | Wave number (per cm) | Energy (ev) |
| Far IR | 300,000 | 30,000 | 0.10 | 333 | 0.041 |
| Near IR | 10000 | 1000 | 3.00 | 10000 | 1.24 |
| Red (Limit) | 7000 | 700 | 4.29 | 14300 | 1.77 |
| Red | 6500 | 650 | 4.62 | 15400 | 1.91 |
| Orange | 6000 | 600 | 5.00 | 16700 | 2.06 |
| Yellow | 5800 | 580 | 5.17 | 17240 | 2.14 |
| Green | 5500 | 550 | 5.45 | 18200 | 2.25 |
| Greeen | 5000 | 500 | 6.00 | 20000 | 2.48 |
| Blue | 4500 | 450 | 6.66 | 22200 | 2.75 |
| Violet | 4000 | 400 | 7.50 | 25000 | 1.30 |
| UV Long | 3660 | 366 | 8.19 | 27300 | 3.39 |
| UV Short | 2537 | 254 | 11.82 | 39400 | 4.89 |
Kurt Nassau and the Fifteen Causes of Color
Causes of Color: Incandescence and Simple
Excitations
Causes of Color: Energy Bands
Causes of Color: Ligand Fields
Causes of Color: Molecular Orbitals and
Charge Transfer
Causes of Color: Physical Optics
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update