Causes of Color: Energy Bands
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
Metals and semiconductors have free electrons, and that dictates their colors and other optical properties.

The diagram above shows some aspects of natural polarization. At top left, a beam of light enters from upper left (only its electric field vector shown). It strikes an electron, causing in to oscillate aned emit light. The light is polarized along the oscillation direction of the wave.
At top right, we see sunlight causing electrons in the air to oscillate. The observer sees any light polarized at right angles to the plane of the diagram, but he also sees light polarized in the plane of the diagram. The exception is light scattered from the direction perpendicular to the Sun. Since that electron is oscillating toward and away from the observer, he sees only the light polarized perpendicular to the plave of the diagram. So sky light is polarized, and photographers use polarizing filters to deepen the color of the sky and enhance contrast. In principle, the light should be completely polarized, but there's always a certain amount of additional scattering, so the polarization is incomplete.
At bottom is light reflecting off a surface. The incoming light is polarized both parallel to and perpendicular to the plane of the diagram. When it strikes the surface, it causes electrons to oscillate, but oscillations along the line of sight are not seen, and only transverse oscillations are visible. Thus, reflections off non-metallic surfaces are polarized. Polarizing sunglasses make use of the fact that most glare-producing surfaces are horizontal (car hoods, pavement, water) and have polarizers oriented to block such light.
Metals
Metals basically consist of positive ions surrounded by an electron "gas." The electrons are all part of a single system, and essentially identical, and by the rules of quantum mechanics, only two of them can occupy any energy level. So the electrons fill every possible energy level from zero up to some maximum. Above that maximum are innumerable empty energy levels, so any imaginable photon can always find an electron and an energy level to excite it to.
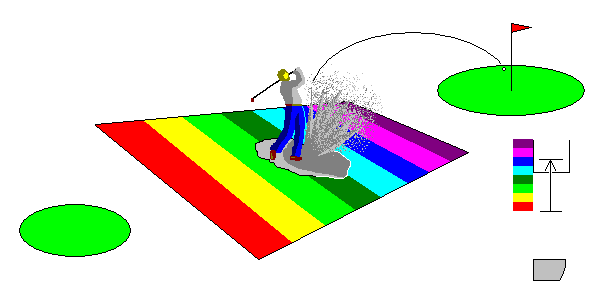
The cartoon below illustrates the physics. The tee is the energy level of an electron. The colored bands indicate the energy of visible light wavelengths. The green (golf, not color) represents the conduction band.

In the lower right of each figure is a more conventional model. The electrons can be pictured as filling a bowl up to some maximum energy, the Fermi Surface. The bowl, where electrons are bound to atoms, is called the valence band. Above that is a possibly empty set of energies that can be occupied by free electrons, the conduction band.
 |
In metals,
it's basically a putting green. A mere putt can get from the ground state of the electron to any
visible light energy, so any photon can excite an electron to an energy
level in the visible range. You could also drive the ball far outside that
range (the ultraviolet) and that would work, too, but you can't see it. At lower right, the condutcion band is immediately above the valence band and any photon can find an electron and an energy level to excite it to. |
The abundance of free electrons in metals means that light can't get even a wavelength deep before being absorbed. The light causes electrons to oscillate and emit light of exactly the same properties as when it entered. Thus, metals are highly reflective and the reflected light is not polarized.
Pure Semiconductors
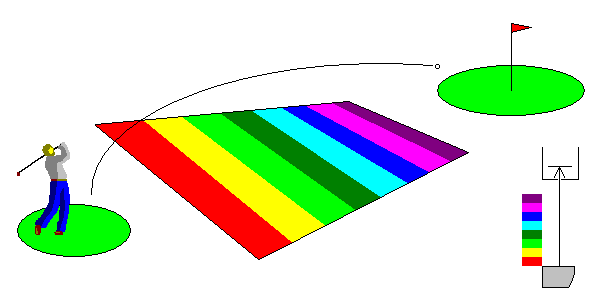
Some materials have all their electrons bound to atoms and they don't normally conduct electricity. But there are empty energy levels above that where free electrons can exist. If you excite electrons strongly enough, the material can conduct electricity. Such materials are termed semiconductors. Our ability to control this process is responsible for solid state electronics.
At lower right, we see that there is no energy in the visible range capable of crossing the gap. The material doesn't interact with visible light and is transparent.
 |
In the case above, it takes energy well above visible light energies to reach the so-called conduction band. No visible photon will interact with the material at all, and it's colorless. Diamond is an example. The gap between the valence band, where electrons are bound to atoms, and the conduction band is quite wide, and such materials are termed wide band-gap semiconductors. Zinc sulfide, titanium dioxide and tin oxide are other examples. In nature, those minerals (sphalerite, rutile and cassiterite, respectively) are often dark due to iron impurities. |
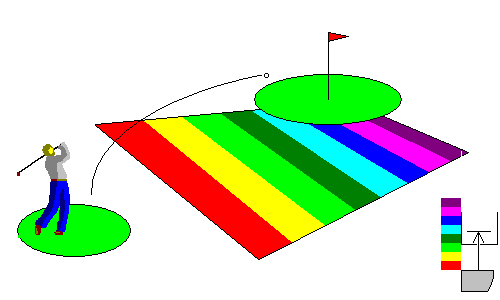
In this situation, photons in the blue and violet energy range can hit the conduction band. The material is transparent to long wavelengths but absorbs blue and violet. These materials, medium band-gap semiconductors, tend to be transparent and brightly colored red or yellow. Examples include sulfur, mercury sulfide (cinnabar) and arsenic sulfides (orpiment and realgar). Cadmium sulfide (the mineral greenockite) is a common pigment because of its brilliant yellow color (cadmium yellow).
At lower right, we see that photons at the blue end of the spectrum can cross the gap, and they are absorbed, but those at the red end are not.
 |
Orpiment and realgar (upper left), cinnabar (upper right) and sulfur (bottom) absorb the blue end of the spectrum and transmit yellow, orange or red. |
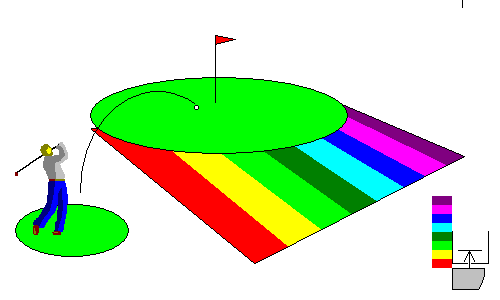
In this situation, the material still has no free electrons but a mere chip shot can kick an electron into the conduction band. Since any wavelength of visible light can do it, these materials look metallic, like the minerals galena (lead sulfide) and pyrite (iron sulfide). In fact most metallic sulfides are in this group. At lower right, we see that the gap between the valence band and the conduction band is so small almost all visible photons can bridge it.

Doped or Impure Semiconductors:
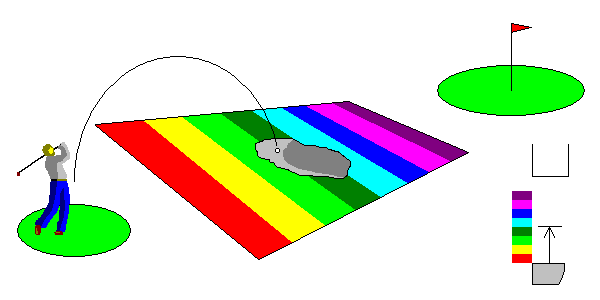
In the case above, you can't make the hole using any energy in the visible range. But you can hit the sand trap. An impurity atom that makes a semiconductor capable of interacting with radiation that otherwise wouldn't affect it is called an acceptor. You might guess from the cartoon that low-energy wavelengths would not interact with the acceptor. Boron impurities in diamond are acceptors and result in blue color like the famous Hope diamond.
At lower right, the gap between the valence and conduction bands is too wide for visible light to cross it, but an impurity atom provides additional energy levels that are accceeible.
Impurity atoms that can supply electrons to cross a band gap are called donors. Nitrogen impurities in diamond are acceptors and result in yellow color. At lower right, a donor atom can supply electrons that can cross the gap to the conduction band. The energy levels begin at the donor atom since those are the energies we're concerned with.
Although diamonds are the most commonly cited natural cases, artificially doped semiconductors are the backbone of solid state electronics.
Color Centers
The term "color center" is sometimes misapplied to an irregularity in a crystal that can interact with visible light. Color centers are more complex than merely having an impurity atom someplace. Substituting, say, manganese into other minerals to create fluorescence, like the spectacular minerals of Franklin, New Jersey, is pretty much like any other transition metal color. Mn++ substitutes for Ca++ or Zn++, and the ionic radii and charge balance work out, so there's no real problem.
But you can't, say, stick a free electron into a gap in a crystal structure because it would create a charge imbalance. In all probability, nothing big enough to see with a microscope has perfect charge balance, but the imbalance has to be pretty small, and definitely not enough to affect color. After all, when you walk across a rug and touch a doorknob, you discover you're packing a pretty good charge imbalance, but it doesn't affect your body chemistry in the slightest.
Color centers are charge irregularities within crystals that interact with visible light. Electron centers are places where electrons are found where electrons are not normally found. Hole centers are places where elctrons are missing from locations they would normally occur. Usually they occur in pairs because of the need to maintain overall charge balance
 |
The common purple color of fluorite is most often due to color centers where a fluorine atom is missing and an electron is trapped in the space. |
 |
Smoky quartz forms when quartz contains a small amount of
aluminum.Protons occupy interstitial spaces to make up for the
fact that Al only has a +3 charge. Irradiation strips an
electron off an oxygen atom to create a hydrogen atom, and the
depleted oxygen atom becomes a color center. A similar process involving Fe+++ instead of aluminum accounts for some amethysts. |
Kurt Nassau and the Fifteen Causes of Color
Atoms and Light
Causes of Color: Incandescence and Simple
Excitations
Causes of Color: Ligand Fields
Causes of Color: Molecular Orbitals and
Charge Transfer
Causes of Color: Physical Optics
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update