Causes of Color: Physical Optics
Steven Dutch, Professor Emeritus, Natural and Applied Sciences, University of Wisconsin - Green Bay
In this class of color causes, the color is not created by interaction with the electrons of the material, but by the mechanical interactions of the light with the material.
Dispersive Refraction
In most materials, the index of refraction varies with wavelength so that colors are separated when they cross an interface obliquely. In most cases, the separation, or dispersion, is not enough to be obvious, for example when looking into a pond or through a window. But under some circumstances, the dispersion is dramatic.
Rainbows

A rainbow is created by a cone of raindrops that disperse light and reflect it back to the viewer. Because the raindrops are falling, you never see the same rainbow for more than an instant. Also, because different observers will see different drops, no two people ever see the same rainbow.

All that matters is the geometry. Light from, say, a nearby waterfall and from a distant rainstorm will form the same rainbow.

If a rainbow is only created by geometry, then can a rainbow be reflected off, say, a lake? Surprisingly, yes. The drops that form the "direct" rainbow are different from those that create the reflected rainbow. In the latter case, the light is reflected off the water and then to your eye.

As is well known, there are often two rainbows. The second bow is produced by light reflecting twice within a drop. Light from the primary bow is reflected back 138 degrees or 42 degrees from the anti-solar point. Light from the secondary bow is reflected 231 degrees, or 51 degrees from the anti-solar point. The colors are reversed in the secondary bow, and the right side of the primary bow corresponds to the left side of the secondary bow. The secondary bow is nine degrees from the primary bow and outside it.

Can there be additional rainbows? Yes, but don't expect them to be just outside the secondary bow. The third and fourth-order bows are back in the direction of the sun, where there is a great deal of scattering, and they nearly overlap. I've tried quite a few times to see tertiary rainbows and never succeeded.
 |
Rainbows are one of the best known examples of dispersion. |
 |
A fogbow and glory from the rail of a ship in Alaska. The fog is
drippy enough that the droplets give appreciable dispersion, plus
impressive supernumerary arcs. A much smaller glory is visible at
the center of the fogbow arc. Fogbows don't generally show color because the tiny fog droplets diffract light and smear out the colors. This was a very wet fog with large droplets. |
Haloes

Haloes around the Sun and Moon are most often made by ice crystals. Light that enters a crystal is dispersed as in a prism. In addition, light can be preferentially reflected off crystal faces. The predictable geometry of ice crystals (face orientations, not exact shapes) means halo phenomena can be accurately modeled in great detail.

This diagram shows some of the more common ice halo phenomena. The 22-degree halo, sun dogs, sun pillars and upper tangent arc are the most common, the 46 degree halo and parhelic circle are less common. In extremely rare cases, very complex displays spanning the entire sky have been seen. The display below shows a 22-degree halo, sun dogs and parhelia, and an upper tangent arc.

 |
This brilliant solar halo and sun dog are created by fine wind-blown snow. |

Water droplets in front of the Sun or Moon act like a prism and disperse light. There's no interior reflection, so what you see is a colored halo around the Sun or Moon.
 |
You don't need distant clouds or a celestial object to produce haloes. Nearby lights and a foggy window will do nicely. |
Green Flash
The green flash is not a myth, and not an optical illusion, nor an urban legend. It is real and can be photographed. It occurs because the atmosphere can disperse light. Usually the blue wavelengths are absorbed or scattered before they reach the eye.
 |
 |
I have managed to photograph the green flash twice. The above photos were taken in February 2019, over the Atlantic Ocean. It really is green, not manipulated.
The atmosphere does have some dispersion, because of its density gradient. Red light is bent more than blue. When the last pinprick of sunlight disappears, red and yellow have been refracted into the ground before reaching the observer. Blue and violet still have not reached the observer, and are mostly scattered and absorbed anyway. Under very favorable circumstances, the last bit of visible sunlight will be green. It requires very clear air, an absence of red colors (indicating little scattering) and a sharp horizon. High latitudes are best because the sun sets at a grazing angle and the green flash lasts longer. There's probably a visual effect as well, since the green is enhanced by contrast with the surrounding red sunset colors.

Fire in Gems
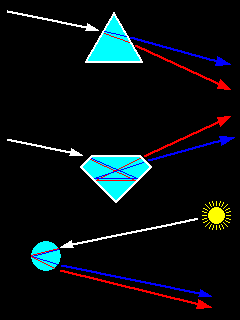 |
Top: Dispersion of light by a prism Middle: Dispersion of light in a gemstone. Facet angles are selected for maximum reflection and dispersion Bottom: Dispersion in a raindrop to form a rainbow. |
Scattering
The blue of the sky is caused by Rayleigh Scattering. Rayleigh scattering is inversely proportional to the fourth power of wavelength, so it is far more effective at the blue end of the spectrum. Why the sky is blue, and not purple, depends on a whole array of factors, including:
- The sun emits peak radiation in the green part of the spectrum, and emits more blue than violet light.
- Our eyes are most sensitive to green light (not an accident).
- Short wavelengths are absorbed more by the atmosphere.

Why the sky is blue: Red, yellow and green pass straight through with little scattering. Blue is abundant and gives the best combination of penetration plus scattering. Violet has even more scattering, but there is less of it, and also it is absorbed to a greater extent.
Rayleigh scattering polarizes sky light, and the polarization is at a maximum 90 degrees from the Sun. But multiple scattering randomizes the light, so light from clouds is not polarized. Photographers take advantage of this effect to darken the sky and improve contrast with clouds.
Sunset colors are at the red end of the spectrum because the sunlight passes through a longer thickness of sir. When the sun is high in the sky, only the red and yellow aimed directly at us is seen. Any red and yellow aimed away from us simply travels on witout being scattered to our eyes. But when the sun is low, blue wavelengths are mostly lost to scattering and absorption, while red and yellow can reach us. Also, there is more scattering of red and yellow to our eyes as well.
So the green flash, above, has a scattering element. The air has to be very clear, allowing green light to reach the viewer with minimal scattering.
Scattering also occurs with particles comparable in size to the wavelength of light, and since the fourth power law of dependence on scattering with wavelength still applies, the scattered light is blue. Such blue coloration is often called Tyndall blue. Many biological blue colors are Tyndall blues, such as blue eyes and blue veins. The blue is enhanced by a dark background, like hemoglobin in veins or melanin in eyes.
Virtually all white materials are white due to scattering. Many references describe Mie scattering, which happens around large particles, and stop there, but that can't be the whole story, because Mie scattering takes place mostly in the forward direction. If Mie scattering were the whole story we'd expect to see clouds pass light and be dark when viewed from the side. Instead, multiple scattering must be dominant. In addition to true scattering, multiple reflections off particles must be important. A sugar bowl is made of grains easily visible to the unaided eye and far bigger than a wavelength of light, and the random mixture of light we see as white has to be due to random reflections of light off crystal faces.
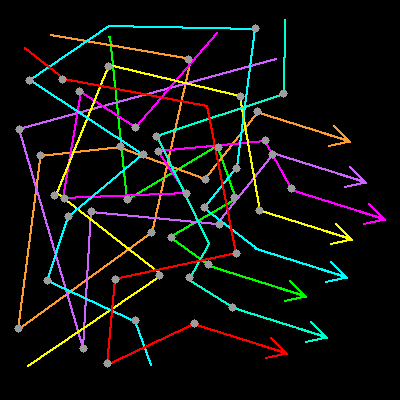 |
Multiple scattering. Light enters a material randomly, scatters,
reflects and refracts off a material, and exits to a viewer as a
uniform mixture seen as white. Note, there's nothing magic about this direction. We cound draw a similar diagram with light exiting in any chosen direction. This diagram just illustrates one possibility. |
A high index of refraction favors multiple scattering because less light refracts into the material and more reflects off it. One very common agent for producting white is titanium dioxide. Interestingly, as an ingredient in inks, it prevents light reflecting off the background and exiting out through the ink. The same material makes white paint white and ink black.
 |
The blue sky is due to Rayleigh scattering, the crepuscular rays to Mie scattering, and the clouds are visible because of multiple scattering. The yellow near the horizon is due to greater absorption of blue by the greater thickness of atmosphere. |
 |
White is the one color that we can assign mostly to a single mechanism. Most whites are due to scattering. And don't give me that nonsense about "white isn't a color." If you can buy it in a bucket of paint, it's a color. |
 |
Clouds are white because of scattering by water droplets or ice crystals. |
 |
Snow is white because of scattering by ice crystals. |
Interference
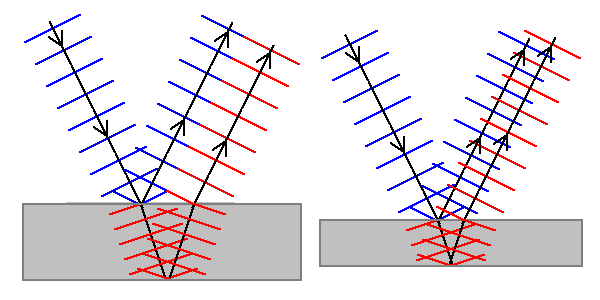 |
When light strikes a thin film, part reflects off the front surface and part off the back. |
For some thicknesses, the two waves reinforce each other or constructively interfere (left). For others (right) they cancel out and destructively interfere.
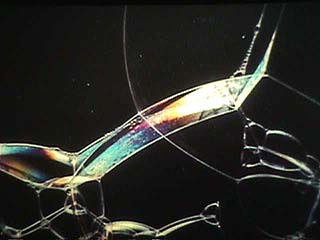 |
The colors on soap bubbles are thin-film interference. Note how there's white at the top, fairly pure colors in the middle, and more dilute hues at the bottom because the film actually tapers from top to bottom. Eventually the film at top will thin out so much the film will break and the bubble will pop. |
 |
Many ore minerals, like chalcopyrite develop thin oxide films on the surface and then exhibit thin film interference. |
 |
iridescent films on metallic minerals are due to a thin oxide layer. |
 |
"Iridescent" colors like this beetle are most likely due to some kind of interference. Diffraction colors would change with direction, but hues that remain constant are mostly interference. |
 |
The brilliant blue of a Morpho butterfly isn't due to diffraction, as often thought, but to interference. |
 |
Peacocks owe their colors to interference. |
Oilfilms on water, and soap bubbles are two familiar examples of thin-film interference. Some ore minerals acquire a thin film of oxides and often display a thin iridescent film. In some cases the film is a distinctive identifying characteristic.
Many biological colors are metallic and brilliant and are sometimes attributed to diffraction, but are actually due to interference. Peacock feathers and the brilliant blue of Morpho butterflies are examples.
Diffraction
Diffraction Gratings
 |
When light hits a boundary of any kind, it sends out a secondary ripple termed diffraction. |
 |
A large number of identical objects creates an array of radiating waves which interfere in certain directions, creating wave fronts (colored lines). |
Diffraction gratings were attempted as far back as the 1700's, but it was the work of Henry Rowland that made them practically possible. Since the grooves had to be ruled mere microns apart, Rowland realized that no possible machine could be rigid enough for the job, and he devised various feedback mechanisms to keep the rulings properly spaced. As he said, he had to regard the entire machine as being made of rubber! Each grating required about 5 km of grooves and took several weeks to complete. Gratings have an immense advantage over prisms in that they are perfectly linear, with a precise geometrical relationship between the wavelength of the light, the groove spacing and the angle of diffraction. Prisms are non-linear and the angles at which light is dispersed are not precisely predictable.
It was Rowland's gratings that enabled the precise measurement of spectral lines that provided the fundamental data for quantum mechanics. Just about every fundamental measurement in quantum mechanics traces back directly to Rowland's work. Rowland's life overlapped the Nobel Prizes, and he ranks high on any list of people who should have gotten one, but didn't.
 |
The play of colors on a compact disc is by now a familiar example of diffraction. The tiny pits on the disk are closely spaced enough to diffract light. |
 |
A distant light viewed through a fine curtain. Diffracted images of the light are visible. |
The Glory
Most observant air travelers have seen colored rings around the shadow of their plane as they climb above low clouds. These rings appear only on water-droplet clouds and they vary in spacing depending on droplet size, so they aren't produced by the same mechanism as rainbows. Since they're circular, they can't have anything to do with the airplane shadow.
 |
The "glory" or "Specter of the Brocken" is a diffraction and interference phenomenon. It appears only on water droplet clouds and the colored rings vary in size with droplet size. Light diffracts off the droplets and then undergoes interference. |
 |
Glories can be seen from the ground, too, if the sun cassts shadows on low fog or cloud banks. Here a glory is visible abound the head of the shadow. If there is a group of observers, each sees a glory around her own head. |
There are several theories for the glory. One attributes it to interference between light reflected off the back of a droplet and light traveling along the surface, as shown below.

Other theories attribute the glory to diffraction off droplets as pinpoint reflectors, followed by interference.
The glory is commonly seen from airplanes, but only flying over water-droplet clouds. Close to the clouds the shadow of the plane appears, but farther away the plane doen't completely block the sun and only a series of colored rings appears. The glory can also be seen by ground observers when a person's shadow is cast onto a nearby cloud bank. If there is a group, each person sees his or her shadow surrounded by a halo. This phenomenon may be the origin of the convention of depicting saints with haloes. It is sometimes called the Specter of the Brocken, after a German peak where it is often seen.
Biological Diffraction
Most of the brilliant, iridescent colors of butterflies and feathers, once attributed to diffraction, are now interpreted as interference. There are a few true diffraction colors, for example, some snake scales. True diffraction colors change color with viewing angle.
Kurt Nassau and the Fifteen Causes of Color
Atoms and Light
Causes of Color: Incandescence and Simple
Excitations
Causes of Color: Energy Bands
Causes of Color: Ligand Fields
Causes of Color: Molecular Orbitals and
Charge Transfer
Return to Mineralogy-Petrology Index
Return to Thin-Section Index
Return to Crystals and Light Index
Return to Crystal Structures Index
Return to Mineral Identification Tables
Return to Professor Dutch's Home Page
Created 22 April 2013, Last Update